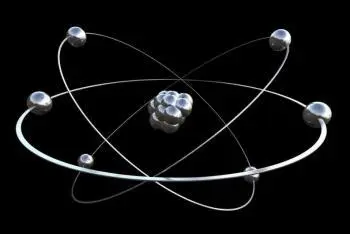
An electron is a stable, negatively charged elementary particle. Along with neutrons and protons, it is one of the three subparticles that make up an atom. For this reason it can also be defined as a subatomic particle. It is part of the lepton group.
They can appear in a free state (without being bound to any atom) or tied to the nucleus of an atom.
The Penning trap is often used to measure these types of particles .
Electrons exist in atoms in spherical shells of various radii. These spherical shells represent energy levels. The larger the spherical shell, the greater the energy contained in this elementary particle.
Electric power
In electrical conductors, current flows are the electrons of atoms that circulate individually from one atom to another in the direction from the negative pole to the positive pole of the electrical conductor. It is what we call electrical energy or electricity.
Although they are usually part of atoms, there are electrons that form beams in a vacuum or move independently through matter.
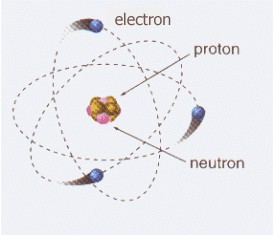 In semiconductor materials, electric current is also produced by the movement of these subparticles.
In semiconductor materials, electric current is also produced by the movement of these subparticles.
Electric charge of the electron
The charge of an electron is approximately -1.602 x 10-19 coulombs (C).
The unit of elementary electric charge is the electric charge of an electron. This elementary charge is the smallest charge found in nature and is considered a fundamental quantity in particle physics and electromagnetic theory.
The electrical charge of the electron is considered negative due to historical convention and the charge of the protons (which is positive) was defined based on the charge of the electrons. This choice of signs has been widely accepted in physics and electronics.
The charge of a proton, which is another elementary subparticle, is equal in magnitude but opposite in sign to the charge of the electron.
Millikan oil drop experiment
The purpose of Robert Millikan and Harvey Fletcher's oil drop experiment was to determine the charge of a single electron. They did this by keeping a small drop of oil floating between two condenser plates.
They discovered that the measured values were always multiples of the same charge. They interpreted this as a charge on a single electron: 1.602 × 10−19 Coulomb.
In 1923 Millikan won the Nobel Prize in Physics.
Electron mass
Its mass is approximately 9.11 x 10-31 kg.
Electrons that move at an appreciable fraction of the speed of light have a greater mass because of relativistic effects. That is, the mass of the electron increases with speed, in accordance with the predictions of Albert Einstein.
Importance of electrons
These fundamental subatomic particles play an essential role in various aspects of physics, chemistry and technology.
Its importance lies in several aspects:
Electricity and electric current
Electrons carry negative electrical charge, and are responsible for electricity and electric current. Electrical current is essential in almost all aspects of our daily lives, from lighting to electronics and power generation.
Conductive materials
 Conductive materials allow electrons to move freely through them. This is crucial in the manufacturing of electrical cables, electronic components and circuits, facilitating the transmission of energy and electrical signals.
Conductive materials allow electrons to move freely through them. This is crucial in the manufacturing of electrical cables, electronic components and circuits, facilitating the transmission of energy and electrical signals.
Chemical bounds
In chemistry, these particles are involved in the formation of chemical bonds between atoms. The sharing and transfer of electrons between atoms is what allows the formation of molecules and chemical compounds, which is fundamental for chemistry and life itself.
Material properties
Electrons determine many properties of materials, such as their electrical conductivity, thermal conductivity, and magnetic properties. These properties are essential in the manufacturing of electronic devices, magnetic materials, and more.
Radiation and spectroscopy
In physics, electrons are involved in radiation processes, such as the emission and absorption of light. Spectroscopy, which uses the interaction of electrons with electromagnetic radiation, is used to analyze the composition of substances and understand astronomical phenomena.
Electronics and technology
Electronic devices, such as computers, cell phones, televisions, and more, operate through the manipulation of electrons. Advances in electronics have transformed modern society and technology.
Particle physics
In particle physics, electrons are fundamental particles studied in particle accelerators. Understanding their behavior has led to the development of fundamental theories about the structure of matter and fundamental interactions.
History and discovery
The discovery of the electron is mainly attributed to JJ Thomson, a British physicist, in the late 19th century. In 1897, Thomson conducted experiments using a cathode ray tube, a device involving electric current and discharges in a partial vacuum.
Thomson observed that there were tiny particles with a negative electrical charge moving from the cathode towards the anode inside the cathode ray tube. These particles were called "electrons" by Thomson. His experiments demonstrated that electrons were basic constituents of matter and had a specific relationship between their charge and mass, which revealed their existence as subatomic particles.
Thomson's atomic model is the first atomic model in which the existence of electrons in the composition of an atom is mentioned.
This discovery was revolutionary and laid the foundation for a new understanding of atomic structure and electrical interactions at the subatomic level. In addition, it paved the way for later developments in physics, chemistry and technology, including modern electronics and quantum theory.


