Osmosis is an intriguing phenomenon that plays a fundamental role in biology, chemistry, and everyday life.
At first glance, it seems like a simple process, but understanding it reveals an underlying dance of molecules that has a profound impact on a wide range of applications.
What is osmosis?
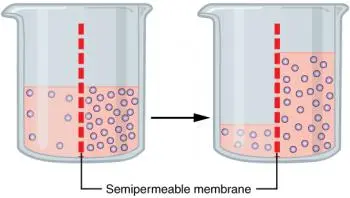
Osmosis is a natural process of water diffusion through a semipermeable membrane. This membrane allows water to pass through, but does not allow larger or charged solutes to pass through as easily.
The driving force behind osmosis is the solute concentration gradient, that is, the difference in the concentration of solutes on both sides of the membrane.
When there is a higher concentration of solutes on one side of the membrane than on the other, water tends to move from the area of lower concentration to the area of higher concentration.
This flow of water stops when an equilibrium is reached in the concentrations of solutes on both sides of the membrane. Osmotic pressure is the pressure needed to stop this flow and is a crucial property in cell biology and everyday life.
How does osmosis work?
To understand how osmosis works, it is essential to understand the following key concepts:

The difference in the concentration of solutes on both sides of the membrane triggers the process. When there is a higher concentration of solutes on one side of the membrane compared to the other, a concentration gradient is established.
Water tends to move from the region of lower solute concentration to the region of higher solute concentration through the semipermeable membrane. This movement is passive and occurs without the expenditure of energy.
Osmosis continues until an equilibrium in solute concentrations is reached on both sides of the membrane. At this point, the osmotic pressure is equal on both sides of the membrane, and the net flow of water stops.
Osmotic pressure is the pressure necessary to stop the flow of water through the membrane and is directly related to the concentration of solutes. The higher the concentration of solutes in a solution, the higher the osmotic pressure.
Inverse osmosis
 Reverse osmosis is a water purification process that uses a semipermeable membrane to remove impurities and dissolved contaminants.
Reverse osmosis is a water purification process that uses a semipermeable membrane to remove impurities and dissolved contaminants.
Through the application of pressure, water is forced through the membrane, while impurities, such as salts, minerals, heavy metals, bacteria and viruses, are trapped. The result is high-quality purified water that is used in a variety of applications, from drinking water production to liquid concentration in the food industry.
Reverse osmosis is a highly effective method to obtain clean and safe water, contributing to the elimination of contaminants and improving water quality.
Examples of osmosis
Below I present some examples of osmosis in everyday life:
Water absorption by plant roots
In this biological process, plants absorb water and nutrients from the soil through their roots. Osmosis plays a critical role as water flows from the soil into the root cells due to differences in solute concentrations.
Raisins
Raisins are produced by dehydrating grapes. The grapes are soaked in a concentrated sugar solution. During this process, osmosis is evident: the water in the grapes moves toward the solution due to the difference in solute concentration. As a result, the grapes shrivel and dehydrate, creating raisins.
Blood cells and medical dialysis
 Osmosis plays a critical role in medicine, especially in kidney dialysis, a blood filtration process. Dialysis membranes allow water and solutes to pass through, while retaining waste products and unwanted substances.
Osmosis plays a critical role in medicine, especially in kidney dialysis, a blood filtration process. Dialysis membranes allow water and solutes to pass through, while retaining waste products and unwanted substances.
Osmosis facilitates the removal of solutes and balances fluid levels in the body.
Food preservation
In the food industry, osmosis is used to preserve foods such as cured meat, salted fish or ham.
Food is immersed in concentrated salt solutions, causing water to move out of the food into the solution by osmosis. This process dehydrates food and contributes to its preservation.
Water treatment
Reverse osmosis is a key method for water purification.
In this process, water is pressurized and passed through a semipermeable membrane that retains contaminants, salts, and other solutes. The result is purified water that is safe to drink and has various industrial applications.
Animal cell behavior
Animal cells exemplify how osmosis affects their state in different environments.
-
In an isotonic solution, where solute concentrations are equal inside and outside the cell, the cell maintains its shape.
-
In a hypotonic solution, with a lower concentration of solutes outside, water enters the cell, which can lead to swelling.
-
In a hypertonic solution, with a higher concentration of solutes outside, water leaves the cell, which can cause it to shrink.