In 1904, Hantaro Nagaoka developed an atomic model that complemented Thomson's atomic model. Nagaoka's model is also known as the Saturnine atomic model.
The Saturnine model is a hypothetical atomic model of the atomic structure as opposed to Thomson's plum pudding model. In this model the existence of the atomic nucleus was postulated for the first time.
What is Nagaoka's atomic model?
At the beginning of the 20th century, after the studies of the Dalton and Thomson atomic models, physicists had only just begun to understand the structure of the atom. The discovery of the electron demonstrated the existence of negative charges in the atom and this, in order for it to be globally neutral, necessarily implied that there were also positive charges.
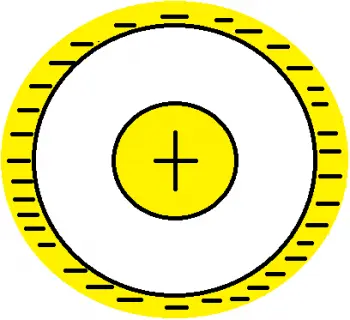
Hantarō Nagaoka, on the basis that opposite electric charges are impenetrable, proposed an atomic model based on a large, massive sphere with a positive electric charge. This sphere was the atomic nucleus and was surrounded by several orbiting electrons. Nagaoka described these orbits as circular orbits, equivalent to Saturn and its rings.
Nagaoka explained atomic stability according to his model with an analogy with the stability of Saturn's rings. James Clerk Maxwell had recently published a study on this model and made two predictions:
- the existence of a very massive core, in analogy with the disproportion between the mass of Saturn and that of the ring;
- that the electrons rotate around the nucleus bound by the electrostatic force, just as the particles of the ring rotate around the planet bound by the gravitational force.
Saturnian model
The Nagaoka model is known as the Saturnian model because Japanese physicist Hantaro Nagaoka proposed a representation of the atom in 1904 that resembled a Saturn-like planetary system. In this model:
- The atomic nucleus is represented by a large, positive sphere, similar to the planet Saturn.
- The electrons are arranged in a rotating ring around the nucleus, like the rings of Saturn.
Nagaoka was inspired by the stability of Saturn's rings to suggest that electrons could be held in circular orbits around the nucleus by attractive and repulsive forces.
How did Nagaoka's model influence Rutherford's atomic model?
Both predictions were sufficiently confirmed by Ernest Rutherford, who mentioned Hantarō Nagaoka's model in the 1911 paper announcing the discovery of the nucleus.
However, Rutherford's atomic model revealed how wrong Saturn's model was. In reality, the core was much smaller than Nagaoka had assumed. Moreover, an electrically charged ring would have been unstable to oscillations in a direction orthogonal to the plane of rotation of the ring.
Rutherford's discoveries would serve as the basis for studies to develop the atomic model of Niels Bohr and the later atomic model of Sommerfeld.
It should also be noted that Nagaoka, through the Saturn model, was unable to predict spectrographic phenomena such as the formation of spectral lines and radioactivity. Following these new discoveries, Nagaoka himself abandoned his model in 1908.
Biography of Hantarō Nagaoka and his contribution to atomic theory
Nagaoka Hantarō (August 15, 1865 – December 11, 1950) was the most prominent Japanese physicist during the late Meiji period. He was one of the founders of the Japanese physicists who began the Meiji period, the founder of the scientific school. Author of various works on electricity and magnetism, atomic physics and spectroscopy.
Born in Ōmura, Nagasaki Prefecture, Nagaoka was educated at the University of Tokyo. After graduating in 1887, he collaborated with British physicist Cargill Gilston Knott on studies of magnetism. In 1893 he moved to Europe, where he completed his training at the universities of Berlin, Munich, and Vienna. In 1900 he attended the First International Congress of Physics in Paris, where he heard Marie Curie lecture on radioactivity, an event that increased Nagaoka's interest in atomic physics.
Nagaoka's main works
Hantaro Nagaoka rejected Thomson's model on the grounds that opposite electric charges are impenetrable and proposed the alternative atomic model explained above. However, he himself abandoned it in 1908.
After abandoning his atomic model, Nagaoka turned to spectroscopy and other fields. In 1909, he published a paper on the inductance of solenoids.
In March 1924, he described experiments in which he claimed to have obtained a milligram of gold and some platinum. The discovery was made by subjecting mercury to an electric field of 15 × 10 6 V/m for a few hours. The experiment was subsequently repeated with contradictory results, a result of contamination from the gold already present in the mercury.
In 1929, Nagaoka detected radio disturbances produced by meteors. The Japanese physicist hypothesized that it would be possible to establish communication between two stations on the ground. To do this, he used the ionized trail left by the meteoroid as a radio link when it entered the atmosphere.