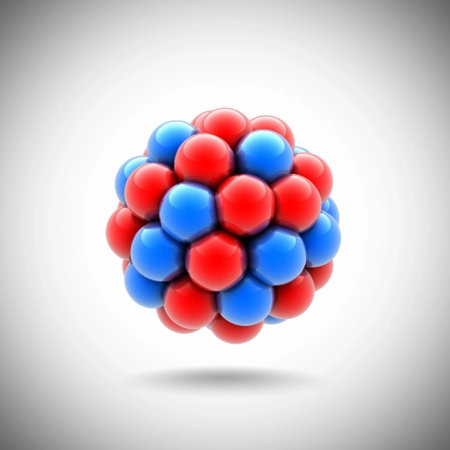
The atomic nucleus is the tiny central region of the atom, with a positive electrical charge, where almost all of its mass is concentrated.
Although it represents only a fraction of the total size of the atom, its influence is crucial in defining the chemical and physical properties of the elements.
Subatomic particles of the nucleus
The nucleus is made up of protons and neutrons, collectively known as nucleons. Protons have a positive electrical charge, while neutrons are electrically neutral. However, in the simplest hydrogen nuclei (hydrogen-1), only one proton is found, with no neutrons.
Each chemical element is defined by its atomic number (Z), which corresponds to the number of protons in the nucleus and determines the total electrical charge of the nucleus. In addition, the mass number (A) is the sum of protons and neutrons, providing an approximate measure of nuclear mass.
Composition of the core and its structure
Almost all of the atom's mass is in the nucleus, since electrons, although essential for chemical interactions, have negligible mass compared to protons and neutrons. The size of the nucleus varies depending on the element: from about 1.75 femtometers (fm) in the nucleus of hydrogen to 15 fm in the heavier elements, such as uranium.
The fundamental numbers that describe the atomic nucleus are:
- Atomic number (Z): number of protons.
- Number of neutrons (N): quantity of neutrons in the nucleus.
- Mass number (A): sum of protons and neutrons, A = Z + N.
Nuclear forces and bonds between protons
The presence of positively charged protons creates a strong electrostatic repulsion within the nucleus. However, a much stronger force, known as the strong nuclear force, holds the nucleons together. This force is short-range but sufficient to counteract the repulsion between the protons.
Nuclear binding energy, which is responsible for holding the nucleus together, is released in nuclear fusion reactions. In these processes, when nuclear bonds are broken, energy is generated according to Einstein's equation:
E=mc 2
This relationship describes how the loss of mass during these reactions is converted into energy.
Examples of atomic nuclei in nuclear energy
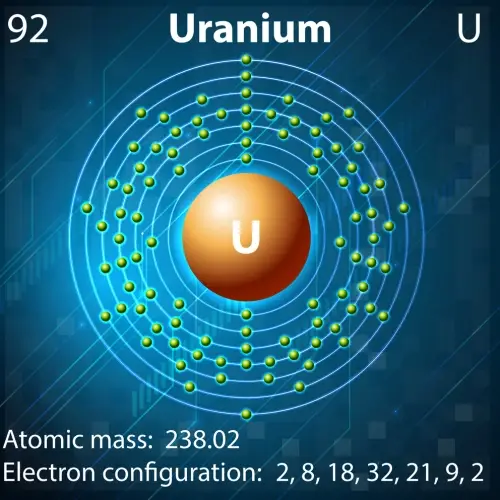 In the field of nuclear energy, some atomic nuclei are essential due to their unique properties:
In the field of nuclear energy, some atomic nuclei are essential due to their unique properties:
- Uranium-235: This isotope of uranium is key to nuclear reactors and nuclear weapons. Its nucleus, made up of 92 protons and 143 neutrons, is highly fissile, meaning it can split into smaller nuclei when bombarded with neutrons, releasing a huge amount of energy.
- Plutonium-239: Produced artificially, it is another crucial nucleus for nuclear fission. It has 94 protons and 145 neutrons, and is used in both reactors and nuclear weapons due to its high capacity to release energy.
- Deuterium (Hydrogen-2): An isotope of hydrogen used in nuclear fusion experiments, where it is combined with tritium (Hydrogen-3) to release clean energy. This reaction is the basis of research into fusion reactors, such as ITER.
Atomic models and the evolution of the theory of the atom
Our understanding of atomic structure has evolved considerably over time. The most significant milestones are highlighted below:
- Dalton's model (1808): John Dalton proposed that all matter is made up of indivisible and invisible atoms. This model did not consider the existence of the atomic nucleus.
- Thomson model (1897): Joseph John Thomson discovered the electron and suggested that atoms have an internal structure. He postulated a model in which negative particles (electrons) are distributed in a positive mass, known as the "plum pudding model".
- Rutherford Model (1911): Ernest Rutherford discovered the atomic nucleus using the famous gold foil experiment. He determined that atoms have a small, dense central nucleus surrounded by electrons.
- Bohr model (1913): Niels Bohr refined Rutherford's model, proposing that electrons orbit the nucleus in defined energy levels. This model introduced quantum concepts in describing electron orbits.
Didactic simile to understand the dimensions
 To understand the proportions of the nucleus in relation to the rest of the atom, imagine a gigantic football stadium, like the Camp Nou, completely empty.
To understand the proportions of the nucleus in relation to the rest of the atom, imagine a gigantic football stadium, like the Camp Nou, completely empty.
In the center of the stadium, we place a small marble. This tiny sphere represents the atomic nucleus, the central part of the atom where almost all of its mass is concentrated. Although it is incredibly small compared to the total size of the stadium, it is the most important piece, as it contains the protons and neutrons that determine the properties of the atom.
The rest of the stadium, all that vast empty space, symbolizes the region where electrons move. Electrons are tiny negatively charged particles that circle around the nucleus like bees hovering around a flower. Although they take up a lot of space, they weigh so little that their mass barely contributes to the total mass of the atom.