In 1904, Joseph John Thomson proposed an evolution of Dalton's atomic model, giving rise to the famous Thomson model, a revolutionary theory that attempted to explain two fundamental properties of atoms at the time.
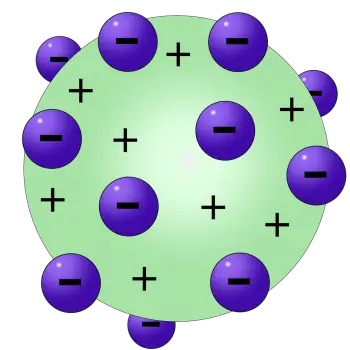
In this model, Thomson postulated that electrons, negatively charged particles, were scattered in a positively charged background, creating a structure resembling a British dessert known as "raisin pudding." This curious nickname comes from the visual analogy of electrons (the "raisins") scattered in a positive background (the "pudding").
This new atomic theory of Thomson attempted to explain two then known properties of atoms:
- Electrons are negatively charged particles.
- Atoms do not have a neutral electrical charge.
Thomson's Postulates
J. J. Thomson's atomic model introduced several key postulates that sought to explain the observed properties of atoms at the time. Here are the key postulates and their features:
- Electrons as subatomic particles: Thomson postulated the existence of negatively charged subatomic particles, which were later identified as electrons. This was an important advance, since until then, atoms were considered indivisible according to Dalton's atomic model.
- Spherical structure of the atom: Thomson imagined the atom as a uniform sphere of positive charge, where the negatively charged electrons were embedded like raisins in a pudding. This concept of a spherical structure contrasted with Dalton's earlier idea of indivisible atoms.
- Uniform distribution of electrons: According to Thomson's model, electrons were distributed uniformly throughout the positively charged sphere. This arrangement sought to explain the electrical neutrality of the atom as a whole.
- Free motion of electrons: According to this postulate, electrons were considered to be able to move freely within the positively charged sphere, which explained the stability of the atom. Thomson suggested that the attractive forces between the electrons and the surrounding positive charge were responsible for keeping the electrons in their orbits.
- Explanation of line spectra: Thomson proposed that electrons rotated freely in ring-shaped orbits within the atom. Differences in the energies of these orbital levels would explain the line spectra observed in the light emission when electrons jumped between these orbits.
Why is it known as the plum pudding model?
 Thomson's model has been commonly compared to the British dessert known as plum pudding, which gave it its distinctive name. Although Thomson did not describe it in this way, the analogy was adopted by others to better illustrate his point.
Thomson's model has been commonly compared to the British dessert known as plum pudding, which gave it its distinctive name. Although Thomson did not describe it in this way, the analogy was adopted by others to better illustrate his point.
In this model, the negatively charged electrons are arranged like raisins embedded in a positively charged mass, which represents the pudding. In this way, the positive charge balances the negative charge of the electrons, keeping the atom as a whole electrically neutral. This simple representation, although now obsolete, helped to visualize how subatomic particles could coexist in a relatively small space, before more precise details about the structure of the atom were known.
Features of Thomson's atomic model
According to Thomson's atomic model, the atom consists of electrons placed in a positively charged "soup", which compensates for the electrically negative charges of the electrons.
According to this model, electrons could spin freely in a droplet or cloud of such a positively charged substance. Their orbits were stabilized within the atom by the fact that when an electron moves away from the center of a positively charged cloud, it experiences an increase in the force of attraction toward the center of the cloud.
This force of attraction brings it back to the center. The force of attraction to the center of a uniformly charged spherical cloud is directly proportional to the distance from its center.
In the Thomson model, electrons are free to rotate in ring orbits, which are stabilized by interactions between electrons. The line spectra were explained by the difference in energies when moving along different ring orbits.
Thomson's model became a precursor to the later Bohr atomic model, which depicts the atom as a likeness of the solar system .
Limitations of the model
Thomson's model of the atom was disproved in an experiment on the scattering of alpha particles on gold foil in 1909, which was analyzed by Ernest Rutherford in 1911. Rutherford's experiment suggested that the atom had a very small nucleus containing a large positive charge.
In 1913, Henry Moseley experimentally demonstrated that the nuclear charge in elementary charges is very close to the atomic number.
This work eventually led to the creation in the same year of the Bohr model, similar to the solar system. According to this model, the nucleus has a positive charge equal to the atomic number and is surrounded by an equal number of electrons in orbital shells.
Thomson's problem
When considering the Thomson model, a still unsolved problem of mathematical physics was formulated - finding the configuration of many charges with the lowest potential energy on a sphere - the Thomson problem.
Thomson's legacy
J. J. Thomson's legacy in physics and chemistry is undeniable, not only for the discovery of the electron, but also for having revolutionized the way we think about atoms. Although the "plum pudding" model was eventually superseded, it represented a crucial step towards the modern understanding of matter. Thomson challenged the notion that atoms were indivisible with his postulates and opened the doors to an era of subatomic research.
In my opinion, what is most admirable about Thomson's work is his ability to look beyond accepted ideas and propose something completely new, which requires remarkable intellectual courage. His model, although simple compared to later advances, was key in the development of modern physics. It is a reminder that every theory, even if it is refined or discarded over time, can be the spark that drives the next great scientific breakthrough.
In this sense, Thomson's legacy lies not only in his atomic model, but in his ability to question the established order and promote a new way of thinking about the nature of matter. Without his work, the current understanding of the atom would not have been possible.