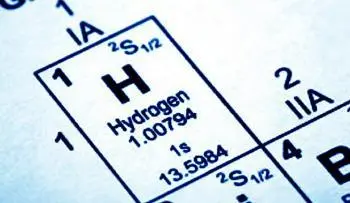
The atomic number indicates the total number of protons in the nucleus of a given atom. It is represented by the letter Z and is placed as a subscript to the left of the element symbol.
Atoms are made up of a nucleus (made up of protons and neutrons) and a shell made up of electrons. Protons are positively charged, neutrons are electrically neutral. Therefore, the atomic nucleus always has a positive charge. On the other hand, electrons have a negative charge.
An atom in its natural state is neutral and has the same number of electrons and protons.
Searching for new items is normally done using this concept. The synthesis of new elements is accomplished by bombarding heavy element atoms with ions. In this way it is achieved that the sum of the atomic numbers of the elements and ionics is equal to the atomic number of the element that is being created.
In general, the half-life of an atom shortens as the number of protons increases.
Importance of the atomic number in the periodic table
The location of the chemical elements in the periodic table depends on the atomic number.
The periodic table is organized into horizontal rows called periods and vertical columns called groups. In it, the elements are arranged in order of increasing number of protons in the nucleus of the atom.
This means that as you go from left to right in a period, the atomic number of the elements increases by one unit. For example, in period 2, lithium has an atomic number of 3, beryllium has an atomic number of 4, and carbon has an atomic number of 6.
The arrangement of elements on the periodic table based on their atomic number also allows one to identify periodic trends in physical and chemical properties. As you move from top to bottom in a group, elements tend to show a gradual increase in their atomic size and ionic radius, as well as a decrease in their electronegativity.
Examples of atomic numbers
Here is a table with some examples of atomic numbers:
|
Element |
Atomic number |
|
Hydrogen |
1 |
|
Carbon |
6 |
|
Oxygen |
8 |
|
Aluminum |
13 |
|
Chlorine |
17 |
|
Potassium |
19 |
|
Iron |
26 |
|
Copper |
29 |
|
Sulfur |
16 |
|
Sodium |
11 |
|
Gold |
79 |
Isotopes and difference with mass number
Isotopes are chemical elements that have the same number of protons (Z) but different numbers of neutrons (N). The chemical properties of isotopes are very different, in some cases.
The mass number (A) of an atom is the sum of the atomic number (Z) and the number of neutrons (N).
Uses and applications
The atomic number has various applications in science and technology.
Next, we discuss some characteristics of atoms related to atomic number and how they are used in physics and chemistry:
Relationship to chemical properties
As the number of protons in the atomic nucleus increases, it influences the way atoms interact with other elements and compounds.
For example, elements in group 1 of the periodic table, such as hydrogen and lithium, have only one electron in their outer shell, making them highly reactive and prone to forming ionic compounds.
Electronic configuration
The atomic number is also essential to determine the electronic configuration of an atom, that is, the distribution of electrons in its different energy levels and sublevels.
As the number of protons is increased, electrons are added at higher energy levels and sublevels. This accumulation of electrons generates periodic patterns and trends in atomic and molecular properties.
Isotope Identification
The atomic number also allows us to identify different isotopes of the same element that have different atomic masses. Isotopes are atoms of the same element that have the same number of protons but differ in the number of neutrons in the nucleus.
For example, carbon-12 and carbon-14 are isotopes of carbon. Although the number of neutrons can vary, the atomic number of carbon is always 6, which distinguishes it from other elements.
Radiometric dating
Radiometric dating is a technique used in geology and archeology to determine the age of samples based on the decay of radioactive isotopes.
By knowing the atomic number of an element, its corresponding radioactive isotope can be identified. Subsequently, by measuring the ratio between the radioactive isotope and its decay products, the age of the sample can be calculated.
Scientific research and development
Knowledge of the atomic number is essential in scientific research, especially in areas such as particle physics, nuclear chemistry, and the synthesis of new materials.
By understanding the atomic number of elements, scientists can study and manipulate the properties and behaviors of atoms and molecules to create new materials with specific characteristics.
Historical evolution of the atomic number
At first, the atomic number was the position in which a chemical element remained when they were arranged in increasing order according to their atomic masses.
In 1913, Johannes H. van den Broek discovered that the number of elementary charges in the atomic nucleus was equal to the atomic number. Later, Niels Bohr adopted this discovery to develop his quantum theory of the structure of atoms and the origin of spectra, which he reflected in the well-known Bohr atomic model.