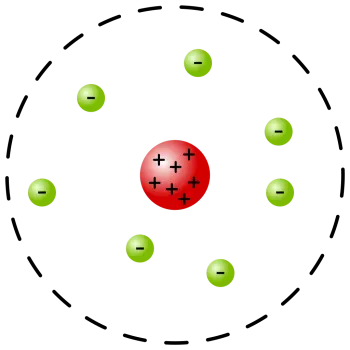
Rutherford's atomic model or planetary model of the atom is a model proposed by Ernest Rutherford.
In 1909 the Geiger and Marsden experiment was performed, also known as the Rutherford experiment, as it was led by Rutherford himself. The Rutherford scattering observed in the investigation suggested that the early "Panettone" and "Saturnian" atomic models were incorrect.
The new model proposed by Rutherford had features that have been maintained even in later models, such as:
-
The concentration of most matter in a small volume compared to the atomic size, that is, an atomic nucleus
-
The presence of electrons revolving around the atomic nucleus.
Rutherford did not say anything about the electrons’ possible movement because he knew that their revolution around the central nucleus would cause electromagnetic radiations. Radiation can occur only when the electron jumps from one orbit to another.
As re showed the atom with a nucleus and electrons orbiting around it, like the planets of the solar system, it became known as the planetary model.
What are the postulates of Rutherford’s model?
-
The atom is mostly empty space. Rutherford denied Thomson's atomic model by confirming the existence of the atomic nucleus, already postulated by Nagaoka. However, Rutherford pointed out that the nucleus of the atom is tiny compared to the atom itself.
-
The atomic nucleus concentrates the mass and the positive charges of the atom, balancing the electrical charge of the electrons.
-
The negatively charged electrons present around the atom do not affect the scattering of the alpha particles.
How did Bohr expand on Rutherford's model of the atom?
Niels Bohr related the line spectra of the elements. He used a hydrogen atom. So, in 1913, Bohr proposed some postulates that altered the view of Rutherford's atomic model. He showed that electrons move around the atomic nucleus in circular paths orbits with well-defined energy (quantum mechanical energy), thus being an energy level or electronic layer. Only specific amounts of energy are allowed for each electron, with multiple integer values of the photon ( quantum number).
He also showed that when all the electrons in the atoms orbit around the nucleus at their lowest energy levels, the atom is in its ground state, which is the most stable. But if the electron absorbs photons, it jumps from a level closer to the nucleus to a higher energy level, more external. It is the activated state or excited state. But it is unstable, and the electron soon emits excess energy, returning to the lower energy level.
Using Max Planck's constant, Bohr obtained an exact formula for the energy levels of the hydrogen atom. Furthermore, he postulated that the angular momentum of the electron is quantized.
What was Ernest Rutherford's experiment like?
In 1911 Rutherford proposed his model of atomic structure to explain the results of Geiger and Marsden's earlier experiment, which indicated the presence of a concentration of positively charged particles in the center of the atom: the atomic nucleus.
However, in his work, Rutherford proposed passing a high-speed α particle through an atom in a gold foil with a positive central charge while surrounded by an equal-magnitude charge of electrons. Rutherford concluded that the charge must be concentrated in a minimal volume relative to atomic size.
He did not attribute any structure to the orbits of the electrons. However, he did mention the Hantarō Nagaoka atomic model.