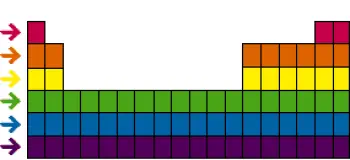
The periodic table of elements is one of the most powerful and fundamental tools in chemistry. In addition to families or groups , the periodic table is also organized into horizontal "periods" or "rows."
Periods have significant importance in understanding the chemistry of elements, as they represent an ordered arrangement of electrons in the electronic shells of atoms.
The structure of a period
Each period of the periodic table represents an additional electron shell in the atoms of the elements as we move from left to right in the table. Starting from the first period at the top, which contains only two elements (hydrogen and helium), the elements in each period have the same number of electron shells.
Examples
For example, in the second period, elements such as lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine and neon all have two electronic shells in their atoms.
In the third period, elements such as sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine and argon have three electron shells, and so on as we move to lower periods.
Properties of elements in a period
Elements within the same period have chemical and physical properties that follow certain patterns and trends:
Atomic size

This is because as protons and electrons are added to the same electron shell, the effective nuclear charge increases, which attracts the electrons more strongly toward the nucleus, reducing the size of the atom.
Electronegativity
Electronegativity, which measures an atom's tendency to attract shared electrons in a chemical bond, tends to increase as we go from left to right in a period.
Elements on the right side of a period are more electronegative than those on the left.
Reactivity
Elements in the same column of the periodic table (in the same group or family) tend to have similar chemical properties because they have the same number of electrons in their valence shell.
However, over a period, as we move from left to right, elements tend to become less metallic and more non-metallic in their chemical behavior.
For example, alkali metals in Group 1 are highly reactive, while elements on the right side of a period, such as fluorine and oxygen, are nonmetals and tend to form covalent compounds.
Ionization Energy
Ionization energy, which is the energy required to remove an electron from an atom, generally increases as we go from left to right in a period.
Elements on the right side of a period have higher ionization energies, meaning it is harder to remove an electron from their atoms.
Importance of periods
 The periods in the periodic table are fundamental to understanding how the electronic structure of atoms influences their chemical behavior. By observing trends in the properties of elements over a period, chemists can predict how atoms will interact and form chemical compounds.
The periods in the periodic table are fundamental to understanding how the electronic structure of atoms influences their chemical behavior. By observing trends in the properties of elements over a period, chemists can predict how atoms will interact and form chemical compounds.
Furthermore, period information is also valuable in inorganic chemistry and compound synthesis.
For example, when seeking to synthesize a new chemical compound, knowing the properties of the elements over the same period can help scientists select the appropriate elements to react in the desired way.
Conclusion
The periods of the periodic table are an essential part of its organization and play a crucial role in understanding the chemistry of the elements.
As we move from left to right in a period, we can observe how the properties and behavior of the elements change systematically due to the addition of electronic shells and the variation in the effective nuclear charge.
This orderly organization of elements into periods is a reminder of the profound relationship between the electronic structure of atoms and their underlying chemistry.