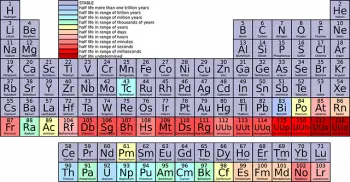
The periodic table of chemical elements is one of the most powerful and essential tools in chemistry and science in general. Among its most distinctive features are "families" or "groups" into which chemical elements are organized according to their similar properties.
Before we dive into the specific families of the periodic table, it is important to understand its general organization:
What are the families of the periodic table?
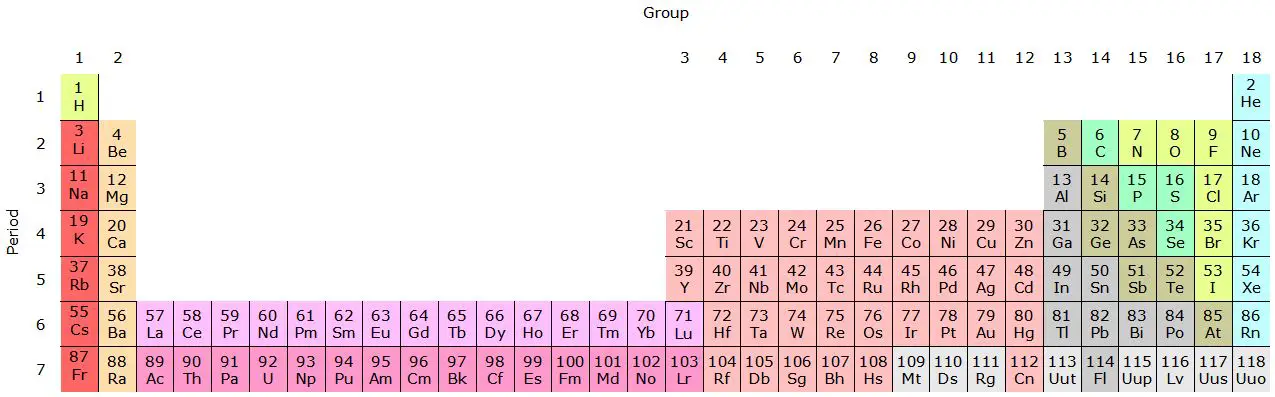 Families in the periodic table are vertical groups of elements with similar chemical properties due to their shared electronic configuration in the outermost electron shell.
Families in the periodic table are vertical groups of elements with similar chemical properties due to their shared electronic configuration in the outermost electron shell.
The periodic table is divided into horizontal rows called "periods" and vertical columns called "groups" or "families." The elements are arranged in increasing order of atomic number, which is the number of protons in the nucleus of an atom.
The elements in each family share similar characteristics and chemical properties. Each family has a specific number and is identified with a Roman numeral or an ordinal number (for example, Group I or Group 1).
Why are elements organized into families?
In the 1860s, Dmitri Mendeleev was attempting to arrange the known elements based on their properties and atomic masses.
During this process, he noticed that when elements were arranged in rows in increasing order of their atomic masses, repetitive patterns of chemical properties similar to each certain number of elements were produced.
This led him to the idea that elements should be organized into columns or families, where elements in the same column would have similar chemical properties.
The most important families of the periodic table
Family 1: Alkali metals
 Alkali metals are the elements in group 1 of the periodic table, which includes hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs ) and francium (Fr).
Alkali metals are the elements in group 1 of the periodic table, which includes hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs ) and francium (Fr).
Basic properties
These elements are highly reactive and tend to lose an electron to form positive ions. They are known for their ability to react vigorously with water and produce hydrogen gas.
Use and applications
Alkali metals find applications in diverse areas, from lithium-ion batteries in electronic devices to glass manufacturing and the synthesis of chemical compounds.
Family 2: Alkaline earth metals
The family of alkaline earth metals comprises the elements of group 2 of the periodic table, which includes beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radius (Ra).
Basic properties
These elements are also metals, but they are less reactive than alkali metals. They have two electrons in their outermost shell and tend to lose those two electrons in chemical reactions.
Use and applications
Alkaline earth metals are essential for the structure of bones and teeth (calcium), the manufacture of metal alloys (magnesium) and nuclear technology (radium).
Family 17: Halogens
 Halogens form family 17 of the periodic table and include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Halogens form family 17 of the periodic table and include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Basic properties
These elements are highly reactive and tend to gain an electron to form negative ions. They are known for their ability to form ionic compounds with alkali and alkaline earth metals.
Use and applications
Halogens find applications in water disinfection (chlorine), pharmaceutical manufacturing (iodine), and photography (silver bromide).
Family 18: Noble gases
The noble gas family, also known as inert gases, includes helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Basic properties
These elements are known for their low chemical reactivity because they have a completely filled electron shell.
Use and applications
This stability makes noble gases useful in applications such as neon lamps, cryogenic refrigeration, and signal lights.
Lanthanide and actinide family
At the bottom of the periodic table, we find two series of elements known as lanthanides and actinides. Lanthanides are f-block elements, while actinides are f-block elements.
The lanthanides and actinides are two series of elements located at the bottom of the periodic table, and are often excluded from the typical group or family numbering of the main table. These series are known as "internal series" or "f series." These series of elements are often called "rare earths" because of their relative rarity in the Earth's crust.
Although lanthanides and actinides are not traditionally numbered as groups on the periodic table, they are sometimes assigned Roman numerals or referred to as "Group 3" for the lanthanides and "Group 4" for the actinides as a simplified way of referring to them. .
Use and applications
Lanthanides are used in the manufacture of powerful magnets, chemical catalysts and in the electronics industry. On the other hand, actinides, including elements such as uranium and plutonium, are of great importance in nuclear technology and energy generation.
Other Families
In addition to the families mentioned above, there are other groups of elements in the periodic table with unique characteristics and properties.
For example, group 3 contains the "light lanthanides" and group 16 contains the "chalcogens," which include oxygen, sulfur, and selenium.