Chemistry is a science based on the organization of chemical elements in the periodic table, an essential tool for understanding and predicting the behavior of atoms and molecules.
Throughout history, several scientists contributed to the development of the periodic table, and one of the first attempts to organize the elements was the law of octaves.
Historical origins
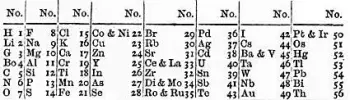
 The law of octaves is a concept that originated in the mid-19th century, when British chemist John Newlands noticed a trend in the chemical properties of elements.
The law of octaves is a concept that originated in the mid-19th century, when British chemist John Newlands noticed a trend in the chemical properties of elements.
In 1864, Newlands proposed his idea in an article titled "On the Law of Octaves." In this work, Newlands observed that when elements are arranged in order of their increasing atomic masses, the chemical properties appear to repeat every eight elements.
This repetition resembles the repetition of musical notes in a musical octave, hence the name "law of octaves."
Limitations of the law of octaves
Although the law of octaves was an important step toward organizing the elements, it had significant limitations.
The main limitation was that it only worked for some of the elements known at the time. In other words, the law was not applied consistently to all elements. Newlands could only apply his law to the first 56 elements of the periodic table, and after that, the repetition of chemical properties was no longer evident.
In addition, Newlands also made mistakes in the organization of some elements. For example, he placed iron and cobalt in the same column, although their chemical properties are strikingly different.
Mendeleev's periodic table: overcoming the law of octaves
Despite its limitations, the law of octaves laid the foundation for the later creation of the modern periodic table. The Russian chemist Dmitri Mendeleev, a contemporary of Newlands, was working on a similar organization of the elements and developed his own version of the periodic table in 1869.
Mendeleev's most important contribution was to recognize that the organization of elements had to be based on their atomic masses, but also had to take into account their chemical properties and the periodicity of these properties.
Organization of the Mendeleev table
Mendeleev organized the elements based on their increasing atomic mass, but left empty spaces in the periodic table where he believed elements unknown at the time should be.
What was surprising was that the chemical properties of the unknown elements perfectly fit Mendeleev's predictions when they were subsequently discovered.
Osolescence of the law of octaves
The success of Mendeleev's periodic table and its ability to predict the properties of as yet undiscovered elements made Newlands' law of octaves obsolete and considered insufficient to explain the organization of the elements.
The modern periodic table and the organization of the elements
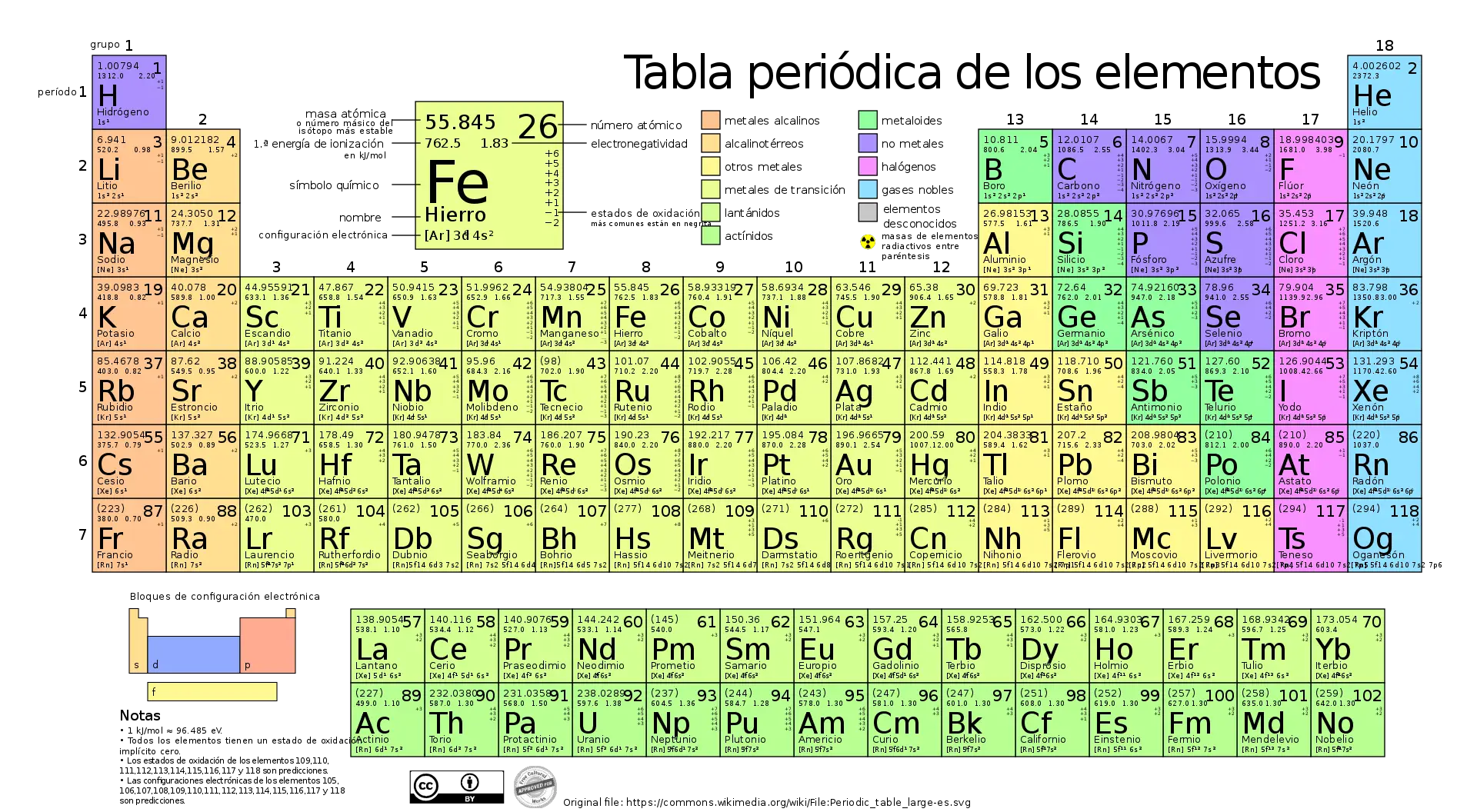 The modern periodic table is based on the structure and organization proposed by Mendeleev, but has evolved over time as more elements have been discovered and progress has been made in understanding atomic structure and chemical properties.
The modern periodic table is based on the structure and organization proposed by Mendeleev, but has evolved over time as more elements have been discovered and progress has been made in understanding atomic structure and chemical properties.
In today's periodic table, elements are arranged based on their atomic number, which is the number of protons in the nucleus of an atom. This results in an organization that clearly shows the periodicity of the chemical properties of the elements.
The horizontal rows, called periods, represent energy levels in which electrons can be found, and the vertical columns, called groups, have elements with similar chemical properties due to their similar electronic configuration.
Conclusions
The law of octaves was an early and valuable attempt to organize the chemical elements, but it had important limitations and could not adequately explain the organization of all the elements.
It was Mendeleev's periodic table that finally overcame these limitations and became the essential tool we know today.
The organization of elements in the modern periodic table is based on atomic number and accurately reflects the periodicity of the chemical properties of the elements, which has been instrumental in advancing chemistry and the understanding of matter at the atomic level. .
In summary, the law of octaves was an important step on this path, but it was only the beginning of an ongoing process of discovery and organization in chemistry.