Schrödinger's atomic model was developed in 1926. Developing the ideas of de Broglie, The Austrian physicist Erwin Schrödinger created wave mechanics or quantum mechanics.
The quantum mechanical model of the atom started from the Schrödiger equation. This mathematical equation revealed the probability of finding an electron at a certain point in an atom. Currently, there is no more precise model of the structure of the atom. For this reason, we also refer to it as the current atomic model.
Until that time, electrons were considered to only rotate in circular orbits around the atomic nucleus. However, Schrödinger claimed that electrons could also turn into more complex elliptical orbits and calculated relativistic effects.
The solutions to the Schrödinger wave equation are of high complexity and are also known as wave functions. The wave function gives only the probability of finding an electron at a given point around the nucleus.
The current atomic model was developed by Schrödinger and Heisenberg based on particle-wave duality.
What limitations does Bohr's atomic model have?
The belief that the atom was composed of a positively charged nucleus surrounded by negatively charged electrons was maintained until 1932.
Bohr's atomic model was a good fit for the hydrogen atom. However, applying the same model to other atoms with higher atomic numbers the energy of electrons at the same level varied.
This energy variation was unexplained in Niels Bohr's model, and therefore it was necessary to correct the model.
The correction proposal was to consider that there were other sublevels within the same energy level. These levels arose naturally by adding relativistic corrections and elliptical orbits.
The discovery of the neutron came in 1932 by James Chadwick. The appearance of this new atomic particle brought scientists closer to a more realistic model of the atom.
To solve the Schrödinger equation, you need to quantify the energies of the electrons. On the other hand, in Bohr's model, these quantum numbers were assumed without a math basis.
Characteristics of the atomic model of Schrödinger
Initially, the Schrödinger model considered that electrons acted like matter waves. In this way, the equation presented by Schrödinger indicates the evolution of this material wave in space and time.
Later, the German physicist Max Born made a probabilistic interpretation of the wave function of electrons. However, the momentum and location of the electron could not be known at the same time. It was due to the Heisenberg uncertainty principle.
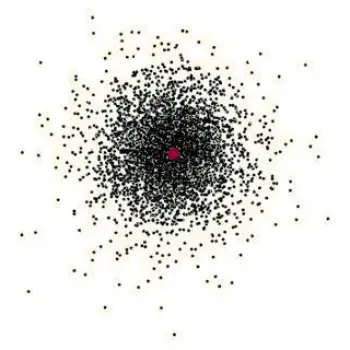
We can represent this new model as a cloud of points (electrons) around the atom’s nucleus. In this electron cloud, the probability of finding the electron increases with the density of points. In this way, Schrödinger first introduced the concept of sub-energy levels.