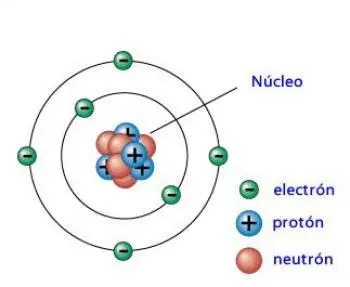
A neutron is a subatomic particle that is part of the atom. The atoms are compounds of protons, neutrons, and electrons. Neutrons and protons make up the atomic nucleus and are also called nucleons. According to the current atomic model, electrons orbit the nucleus.
The number atomic is the number of protons. The mass number is the number of protons and neutrons in the nucleus. The different number of neutrons in the nucleus does not imply the variation of the atom itself. However, it does determine the isotope to which it is a part.
Initially, it was believed that it was an elementary particle, but later it was discovered that it is made up of quarks. Specifically, it comprises three quarks, one up and two down.
The neutron is a 1/2 spin particle; it is a fermion. However, for many years after discovering the neutron, its exact turn was ambiguous. Although it was assumed to be a Dirac particle of spin 1/2, the possibility that the neutron was a 3/2 spin particle persisted.
As a fermion, the neutron is subject to the Pauli exclusion principle. According to Pauli's exclusion principle, two neutrons can not have the same quantum numbers.
The antineutron is the antiparticle of the neutron. The antineutron was discovered by Bruce Cork in 1956, a year after the antiproton was discovered.
What is the life of a neutron?
The half-life of a neutron outside the nucleus is only about 885 seconds (15 minutes).
What is the mass of the neutron?
The mass of a neutron is 1.67492729 × 10-27 kg.
Its mass cannot be determined directly by mass spectrometry due to the lack of electrical charge. However, it can be deduced by measuring the masses of a proton and a deuteron. A deuteron is the nucleus of deuterium, an isotope of hydrogen with atomic number 1 (a proton).
The mass of this particle is slightly greater than that of the proton.
What is the charge of the neutron?
The total electric charge of the neutron is 0 coulombs.
The practical limit obtained is so close to zero that the neutron is considered to have no charge compared to the proton's charge.
On the other hand, protons and electrons, the other two sub-particles of the atom, are charged. They have the same electrical charge but with different signs. Therefore, a neutral atom has the same amount of protons and electrons.
Who discovered the neutron?
James Chadwick, a British physicist and Nobel laureate, discovered the neutron in 1932.
When Ernest Rutherford raised his atomic model, he gave a lecture to the Royal Society in 1920. In it, he argued that there must be another particle with no electrical charge in the atom's nucleus to hold the protons together.
The first indication of the existence of this new particle occurred in 1930. Walther Wilhelm Georg Bothe and H. Becker discovered that bombarding beryllium with alpha particles produced penetrating radiation. Initially, this radiation was believed to be a type of gamma radiation.
In 1932, James Chadwick proposed an alternative explanation for uncharged particles roughly the same size as a proton.
This new particle was called a neutron because of its neutral electrical charge.
How important are they for nuclear fission?
Neutrons are an essential element to generate chain reactions in a nuclear reactor. Nuclear power reactors obtain energy through nuclear fission reactions.
A nuclear reaction of fission occurs when a neutron hits the nucleus of a uranium atom. Enriched uranium atoms are unstable, and the impact is enough to break them. Two or three more free neutrons are generated in each reaction that can collide with other fuel atoms.
The speed with which the neutrons move and the number of free neutrons in the nuclear reactor's core determine the reactor power. Nuclear power plants have mechanisms to control the number of free neutrons in order to manage the number of fission reactions per unit of time. These control mechanisms are the neutron moderator, the reflector, the control rods, etc.
In nuclear energy, the concept "uranium enrichment" refers to the alteration of the number of neutrons in the atomic nucleus in order to obtain another more unstable uranium atom. This modification implies, therefore, an isotope change.