
Dalton's atomic theory , proposed by the English chemist and physicist John Dalton in 1808, marked a turning point in the development of modern chemistry.
Although ideas about the existence of indivisible particles that make up matter date back to the Greek philosophers Leucippus and Democritus , Dalton was the first to support these ideas with experimental evidence, which consolidated atomic theory as a key tool for understanding chemical reactions and the properties of matter.
Postulates of the Dalton model
 Dalton's atomic theory is based on a series of principles that explain the nature and behavior of atoms, the basic units of matter. The four main postulates are presented below:
Dalton's atomic theory is based on a series of principles that explain the nature and behavior of atoms, the basic units of matter. The four main postulates are presented below:
- Elements are made up of indivisible atoms : According to Dalton, atoms are extremely small, indivisible and indestructible particles that constitute the chemical elements. This principle reflected the idea that atoms cannot be broken down into smaller particles.
- Atoms of the same element are identical : All atoms of a particular element have the same properties, including mass and size. This means that, for example, every oxygen atom is identical to every other oxygen atom.
- Atoms of different elements can combine to form compounds : Chemical compounds are formed by combining atoms of two or more elements in definite, simple proportions. This principle helped explain how elements combine in fixed ratios to create new substances.
- Chemical reactions involve the rearrangement of atoms : In a chemical reaction, atoms are rearranged, but neither created nor destroyed. This reinforces the principle of conservation of matter in chemical reactions, a concept already established by Antoine Lavoisier.
Limitations and errors
 Despite being a significant advance for its time, Dalton's atomic theory was not free of errors and limitations. As physics and chemistry developed throughout the 19th and early 20th centuries, several aspects were discovered that did not fit Dalton's original model:
Despite being a significant advance for its time, Dalton's atomic theory was not free of errors and limitations. As physics and chemistry developed throughout the 19th and early 20th centuries, several aspects were discovered that did not fit Dalton's original model:
- Atoms are not indivisible : One of the main flaws in Dalton's theory is the idea that atoms are the smallest, indivisible particles of matter. In the late 19th century, the discovery of subatomic particles such as electrons, protons, and neutrons showed that atoms are actually composed of even smaller particles.
- Existence of isotopes : Dalton assumed that all atoms of the same element were identical. However, with the discovery of isotopes in the early 20th century, it was shown that atoms of an element can have different masses due to the presence of different numbers of neutrons. For example, hydrogen atoms have three isotopes: protium, deuterium and tritium, which differ in the number of neutrons.
- Atoms can break apart under certain conditions : Dalton postulated that atoms were indestructible, but advances in nuclear physics showed that atoms can break apart through nuclear reactions, such as fission and fusion, or through radioactive decay. These processes allow atoms to release energy or transform into other elements.
- Not all compounds are simple combinations of atoms : Dalton's theory suggested that compounds were simple combinations of atoms of different elements. However, the emergence of more complex compounds and the understanding of molecular structures have shown that interactions between atoms can be much more complex than Dalton imagined.
- Dalton's model does not explain electrical interactions : Although Dalton recognized that atoms could combine to form compounds, he could not explain how these combinations occurred. It was not until the advent of Thomson's atomic model and the discovery of the electron that the crucial role of electrical interactions in the formation of chemical compounds began to be understood.
The ponderal laws of chemistry
Dalton's atomic theory not only provided a new perspective on the nature of matter, but also explained several physical laws known as the ponderal laws of chemistry that scientists had already observed. These laws include:
Law of conservation of mass
The law of conservation of mass, formulated by Antoine Lavoisier in the late 18th century, states that the total mass of substances in a chemical reaction remains constant. In other words, the total amount of matter before and after a chemical reaction is the same.
Dalton's atomic theory provided a logical explanation for this law, since, according to his model, atoms are neither destroyed nor created during chemical reactions, they are only rearranged into different configurations.
Law of definite proportions
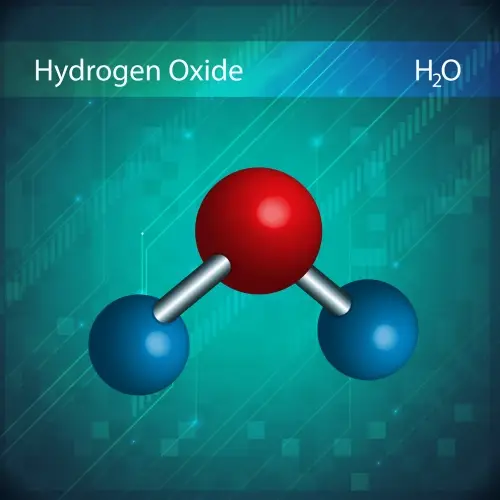 The law of definite proportions, proposed by Joseph Proust in 1799, states that a chemical compound always contains the same elements in the same proportion by mass.
The law of definite proportions, proposed by Joseph Proust in 1799, states that a chemical compound always contains the same elements in the same proportion by mass.
For example, water (H₂O) always has a ratio of 2 grams of hydrogen for every 16 grams of oxygen, regardless of how much water is being analyzed.
Dalton's atomic theory explained this law by suggesting that compounds are made up of atoms of different elements combined in fixed proportions.
Law of multiple proportions
Another fundamental law that Dalton explained with his atomic theory is the law of multiple proportions, which he formulated himself. This law states that if two elements combine to form more than one compound, the amounts of one of the elements that combine with a fixed amount of the other element are in a ratio of simple whole numbers.
For example, carbon and oxygen can combine to form both carbon dioxide (CO₂) and carbon monoxide (CO). In carbon dioxide, there are two oxygen atoms for every carbon atom, while in carbon monoxide, there is only one.
This means that the amount of oxygen that combines with a fixed amount of carbon is in a ratio of 2:1, supporting the idea that atoms combine in simple, definite proportions.
Impact on the development of science
Despite its limitations, Dalton's atomic theory had a profound impact on science, laying the groundwork for the development of modern chemistry. His quantitative approach toward the combination of elements allowed scientists to formulate chemical equations and study reactions in a systematic manner. In addition, Dalton's atomic model was instrumental in the development of other key scientific theories, such as atomic orbital theory , quantum theory , and the Standard Model of particle physics .
Dalton's model also influenced the development of the periodic table of elements, which was organized by Dmitri Mendeleev in 1869, based on the relationship between the properties of elements and their atomic masses. This advance made it possible to classify elements in a consistent manner and to predict the properties of elements that had not yet been discovered.
Evolution of atomic theory after Dalton
Over time, Dalton's atomic theory was refined and replaced by more accurate models.
One of the most significant advances was Thomson's atomic model, also known as the "plum pudding" model, proposed in 1897. In this model, the atom was composed of a positive mass with electrons embedded in it. Although this model was later superseded, it was a crucial step towards the development of Rutherford's atomic model, which introduced the idea of a dense nucleus at the centre of the atom.
In 1911, Ernest Rutherford performed his famous gold foil experiment, which demonstrated that most of the mass of an atom is concentrated in a central nucleus, with electrons orbiting around it. This model radically changed the way we viewed the atom, suggesting that it was not a solid mass, but was composed mostly of empty space.
 Later, Niels Bohr's atomic model, proposed in 1913, improved on Rutherford's model by introducing quantized energy levels for electrons. According to Bohr, electrons could only occupy specific orbits around the nucleus and would emit or absorb energy only when moving between these levels.
Later, Niels Bohr's atomic model, proposed in 1913, improved on Rutherford's model by introducing quantized energy levels for electrons. According to Bohr, electrons could only occupy specific orbits around the nucleus and would emit or absorb energy only when moving between these levels.
Although Bohr's model did not explain all the phenomena observed in the most complex atoms, it was fundamental for the introduction of quantum mechanics in the description of atomic structure.
With the advent of quantum mechanics, the Schrödinger model and the Schrödinger wave equation replaced the idea of fixed orbits with orbitals , which are regions of space where an electron is most likely to be found. Instead of thinking of electrons as particles orbiting the nucleus in defined paths, the quantum model describes electrons as probability waves.
This conception was further advanced with Heisenberg's uncertainty principle , which states that it is not possible to know with simultaneous precision the position and velocity of an electron.
Conclusion
Dalton's atomic theory marked the beginning of a new era in science, providing a solid foundation for the study of matter and chemical reactions. Although his model turned out to be simplified and, in some respects, incorrect, it laid the groundwork for a deeper understanding of atomic structure and the laws governing interactions between elements.
Over time, atomic theory evolved as new discoveries were made. Models such as those of Rutherford , Bohr , and eventually the quantum mechanics of Schrödinger and Heisenberg expanded our understanding of the atom, allowing scientists to explain complex phenomena that Dalton's model could not address.
Today, atoms remain the cornerstone of the study of chemistry and physics. Although technological advances have allowed us to delve deeper into the subatomic structure, Dalton's vision remains relevant in many aspects of the study of matter.
The ability of atoms to combine in definite proportions to form compounds remains a key principle in chemistry, and its theory, although superseded, is recognized as one of the cornerstones of scientific development.
John Dalton's legacy in science is undisputed. His insistence on the importance of empirical observation and his quantitative approach to understanding matter laid the foundations for modern chemistry, and although later discoveries revealed that his view of the atom was incomplete, his contribution remains a milestone in the history of science.