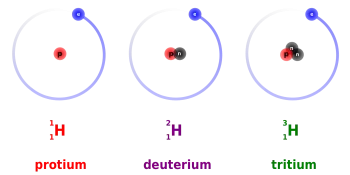
Protium, tritium, and deuterium are the three isotopes of hydrogen. Deuterium and tritium are used as nuclear fuel in nuclear fusion power plants.
Nuclear fusion energy is the union of two light nuclei to form a single one. Fusion occurs when nuclei get so close together that their nuclear forces become very strong. Hydrogen is the element in the periodic table that has the lowest atomic number.
Fusion reactions occur when two atoms with a low mass number join to form a larger atom. This process releases a large amount of energy. Tritium and deuterium are two atoms that can fuse to form helium—the fusion of deuterium and tritium powers the sun and other stars.
The availability of fusion reactors is under study and is intended to be an alternative to current nuclear fission reactors as a source of electrical energy.
The advantage of this technology is that it will produce practically unlimited energy since deuterium and tritium can be obtained from ordinary hydrogen. It should be remembered that this chemical element is the most abundant in the air and seawater.
What is tritium?
Tritium is a radioactive isotope of hydrogen whose nucleus consists of one proton and two neutrons. The most important use of tritium is as a nuclear fuel for obtaining energy through nuclear fusion.
It is usually designated by T, although it should be symbolized as 3H. It was discovered in 1934 by Rutherford, Oliphant, and Harteck in the study of deuterium bombardment with deuterons.
Tritium is a natural isotope generated by the action of cosmic rays on atmospheric gases. Fortunately, exposing the more abundant element of lithium to energetic neutrons can generate tritium.
Several proposals for fusion reactors include a tritium breeding blanket. Among its different uses, it aims to "breed" further tritium fuel, which would be hard to get in sufficient quantities, through the reaction of neutrons with lithium in the blanket.
Tritium has a half-life of 12.3 years and emits very low energy beta (β) radiation (0.018 MeV), totally free of γ radiation, so it has practically no radiotoxicity.
Health Effects of Tritium
Beta particles formed by the decomposition reaction of tritium spread in the air only 6.0 mm and cannot even get past the top layer of human skin. However, this isotope presents a radiation hazard when inhaled, absorbed with food, and absorbed through the skin.
What are the uses of tritium?
Tritium can be used in different applications with different objectives:
-
Self-powered lighting: Beta particles emitted from the radioactive decay of small amounts of tritium cause chemicals called phosphors to glow.
-
Nuclear Weapons: This chemical element is used to enhance the efficiency and performance of nuclear fission bombs and the fission stages of hydrogen bombs in a process known as boosting. Nuclear weapons testing can cause environmental damage, as well as health problems for those who are exposed to the radiation.
-
Controlled Nuclear Fusion: Tritium is an essential fuel for controlled nuclear fusion in magnetic confinement and inertial fusion atomic reactor designs.
-
Analytical Chemistry: Tritium is sometimes used as a radiolabel. It has the advantage that almost all organic chemicals contain hydrogen, so it is easy to find a place to put tritium in the molecule under investigation.
What is deuterium?
Deuterium (²H) is a stable isotope of hydrogen found in nature with a ratio of one in 6500 hydrogen atoms. The atomic nucleus of deuterium is composed of a proton and a neutron.
This hydrogen isotope is obtained from heavy water. It is also known as heavy hydrogen and is represented by the symbol ²H or by D. Although it is a hydrogen atom, the physical properties between deuterium and a light hydrogen atom are maximum since the atomic mass of deuterium it's double.
The Earth and other parts of the Solar System contain deuterium. Fusion in stars tends to destroy deuterium, and, on the other hand, there are no known natural creation processes other than primordial nucleosynthesis.
What are the uses of deuterium?
Deuterium is used in nuclear power as a fuel in nuclear fusion processes along with tritium. Its use is explained by its sizeable effective section of the reaction.
On the other hand, in chemistry, this isotope is used as a tracer in molecules to study metabolic changes and chemical reactions because, from a chemical point of view, it behaves similarly to ordinary hydrogen.