Ionizing radiation is the radiation from the electromagnetic spectrum formed by photons or particles that produce ions when interacting with matter. This definition is valid whether they do so directly or indirectly.
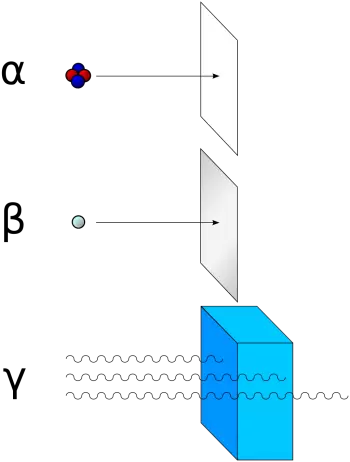
Some examples of ionizing radiation include ultraviolet (UV) radiation of higher energy, X-rays, and gamma rays. Some examples of corpuscular ionizing radiation are alpha radiation and beta decay.
According to the definition of ionizing electromagnetic radiation, optical radiation, visible light, infrared rays, radio frequency (rf), and extremely low frequencies (elf) are excluded.
For health purposes, depending on the type of radiation, exposure to ionizing radiation can cause damage to living tissue. In addition, it can cause mutations, acute radiation sickness, cancer, and death.
Ionizing radiation is invisible and is not directly perceptible by human senses. For this reason, instruments to detect radiation, such as Geiger counters, are needed.
Who discovered ionizing radiation?
Wilhelm Conrad Röntgen discovered these radiations in 1895.
Uses of ionizing radiation
Ionizing radiation is used in various uses, especially in medicine and industry.
In the field of nuclear medicine, the best-known use is X-ray devices. These sources or particle accelerators are used in the diagnosis and treatment (in oncology, for example).
What is the origin of ionizing radiation?
Ionizing radiation can have a natural or artificial origin. Therefore, natural background radiation substances can emit radiation itself. On the other hand, there are artificial generators, such as X-ray generators and particle accelerators.
Some elements are more suitable than others to produce this type of reaction, such as uranium-235. This is because this isotope tends to absorb any neutron that collides with it. When this happens, the uranium-235 ends up breaking into several fragments, releasing other neutrons and nuclear energy.
A normal person is also exposed to low levels of ionizing radiation from the sun, rocks, soil, or other natural sources.
Difference Between Ionizing and Non-Ionizing Radiation
An essential property of electromagnetic fields is frequency and wavelength. Some particles known as quanta of light are responsible for transporting electromagnetic waves. The energy that these waves carry depends on the wavelength; the longer the wavelength, the more energy.
In some cases, the energy carried by these waves is so high that they can tear electrons from an atom and even break molecular bonds. Ionizing radiations have enough energy to carry out these alterations: X-rays, cosmic rays, and gamma rays emitted by radioactive materials.
On the other hand, non-ionizing radiations do not carry enough energy to ionize atoms, so they can not modify atoms or molecule structures. However, some types of non-ionizing radiation can damage the health of people.
Effects of ionizing radiation on safety and health
Ionizing radiation affects biological tissues and health depending on the radiation dose we receive.
The damage that it can cause to biological tissues is of various types and is divided into:
-
Deterministic somatic damage: Deterministic effects involve high doses of radiation over large portions of the body.
-
Stochastic somatic damage: Non-deterministic effects occur at low levels of radiation exposure. In this case, the damage is statistical. Therefore, it is possible to predict the proportion of a given population of exposed people that will be affected. However, it is impossible to know how it will affect each person individually.
-
Stochastic genetic damage: these damages describe heritable genotypic alterations resulting from mutations in genes or germ cell chromosomes.
Somatic damage refers to the damage in the irradiated individual's tissues. On the other hand, genetic damage refers to damage that will affect future generations.
Current anti-pollution regulations set strict limits on individual exposure, including exposure to common building materials such as tuff (which releases radon vapors).
Effects of alpha radiation on health
Alpha particles have a low penetrating power. Therefore, it can be easily stopped by the superficial layer of the skin. In this sense, the skin performs a radiation protection function. Therefore it is not dangerous for humans in cases of external radiation.
Instead, alpha radiation becomes dangerous when the radioactive source is inhaled or ingested because it can directly damage tissues.
Health Effects of Gamma Radiation
On the other hand, gamma radiation (photons), which has a very high penetrating power, can be dangerous for living beings, even in situations of external radiation. The amount of radiation absorbed by a body is called the absorbed dose and is measured in grey.
Examples of ionizing radiation
Some examples of sources of ionizing radiation are the following:
Natural sources:
-
Spontaneous radioactive decay of radionuclides.
-
Fusion nuclear reactions, such as those that occur in the Sun.
-
Nuclear reactions are induced due to the entry into the nucleus of high-energy elementary particles or nuclear fusion.
-
Cosmic rays.
Artificial sources:
-
Artificial radionuclides.
-
Nuclear reactors.
-
Particle accelerators that generate streams of charged particles as well as bremsstrahlung photon radiation.
-
X-ray apparatus as a kind of accelerator, the brake generates X-rays.