
Nuclear fusion is a nuclear reaction in which two light atoms merge to form another, heavier nucleus. The atoms used are isotopes of hydrogen (deuterium and tritium). During fusion, the electrostatic repulsion forces between the nuclei are overcome, and energy is released when a more stable nucleus is formed. This is related to the strong nuclear forces that hold them together.
The energy emitted is so great that it is possible for matter to enter a plasma state.
Nuclear fusion reactions can emit or absorb energy. If the nuclei to be fused have a lower mass than iron, energy is released. Conversely, if the atomic nuclei being fused are heavier than iron, the nuclear reaction absorbs energy. This principle derives from the nuclear binding curve. Energy is released if the reaction product is more stable (higher binding energy per nucleon), and this occurs in nuclei lighter than iron.
To date, a fusion reactor that produces net electrical energy in a continuous and cost-effective manner has not yet been built. All current projects, such as ITER, are experimental. However, several projects—such as the ITER project in southern France—are currently underway with the goal of generating clean energy through fusion.
Examples of nuclear fusion: the Sun
 Examples of nuclear fusion can be found in a variety of situations, both in nature and in human-controlled applications. Some examples include:
Examples of nuclear fusion can be found in a variety of situations, both in nature and in human-controlled applications. Some examples include:
- Fusion in the Sun: The Sun's primary source of energy is nuclear fusion. In its core, hydrogen nuclei combine to form helium, releasing an immense amount of energy in the form of light and heat.
- Hydrogen bomb: Hydrogen bombs, also known as thermonuclear bombs, use nuclear fusion to generate extremely powerful explosions. In these bombs, the fusion of hydrogen nuclei to form helium and other heavy elements releases explosive energy.
- Experimental fusion reactors: Experimental fusion reactors, such as the Tokamak and the Stellarator, have been built to research and develop controlled nuclear fusion as a power source. These devices create conditions similar to those in the Sun's core to achieve fusion.
Requirements of a fusion reaction
To carry out nuclear fusion reactions, the following requirements must be met:
- Achieving a very high temperature to separate the electrons from the nucleus and force the nucleus to approach another nucleus, overcoming electrostatic repulsion forces. The gaseous mass composed of free electrons and highly ionized atoms is called plasma.
- Confinement is necessary to keep the plasma at a high temperature for a minimum amount of time.
- The density of the plasma must be sufficient for the nuclei to be close to each other and be able to generate nuclear fusion reactions.
Confinement for nuclear fusion
The conventional confinements used in nuclear fission reactors are not possible due to the high plasma temperatures they must withstand. For this reason, two important confinement methods have been developed:
-
Inertial confinement fusion (ICF): This involves creating a medium so dense that particles have almost no chance of escaping without colliding with each other.
-
Magnetic confinement fusion (MCF): Electrically charged plasma particles are trapped in a confined space by the action of a magnetic field. The most advanced device is toroidal and called a tokamak.
Operation: Fusion reactions
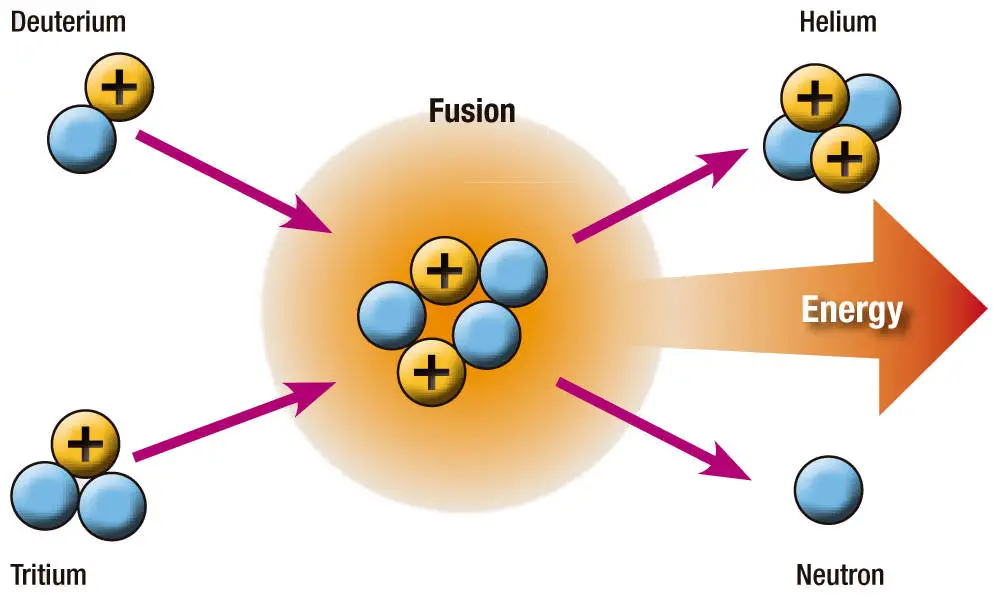
The atomic elements commonly used in nuclear fusion reactions are hydrogen and its isotopes: deuterium (D) and tritium (T). The most important fusion reactions are:
D + T -> 4He + n + 17.6 MeV
By fusing a Deuterium nucleus with a Tritium nucleus, a helium-4 nucleus (with 2 protons and 2 neutrons) is formed and a free neutron is released along with 17.6 MeV of energy.
D + D -> 3He + n + 3.2 MeV
By fusing two Deuterium nuclei, a Helium nucleus is obtained consisting of one neutron and two protons, releasing one neutron and 3.2 MeV of energy.
D + D --> T + p + 4.03 MeV
By fusing two Deuterium nuclei, a Tritium nucleus, a proton and 4.03 MeV of energy are obtained.
For these reactions to occur, the nuclei must be supplied with the kinetic energy necessary to bring them closer to the nuclei to be fused, thereby overcoming the forces of electrostatic repulsion. This requires heating the gas to very high temperatures, such as those assumed to occur in the cores of stars.
The requirement for any nuclear fusion reactor is to confine such a plasma at a high enough temperature and density for just the right amount of time to allow sufficient nuclear fusion reactions to occur, while preventing particles from escaping, to achieve a net energy gain.
This energy gain depends on the energy required to heat and confine the plasma being less than the energy released by nuclear fusion reactions. In principle, 335 MJ can be obtained from each milligram of deuterium-tritium. However, it must be taken into account that the actual efficiency of the system will be lower, and that not all of this energy can be usefully recovered.
Nuclear fuel for fusion
Nuclear fusion reactions require light nuclei. Deuterium and tritium, two isotopes of hydrogen (the lightest element on the periodic table), are primarily used.
1. Deuterium
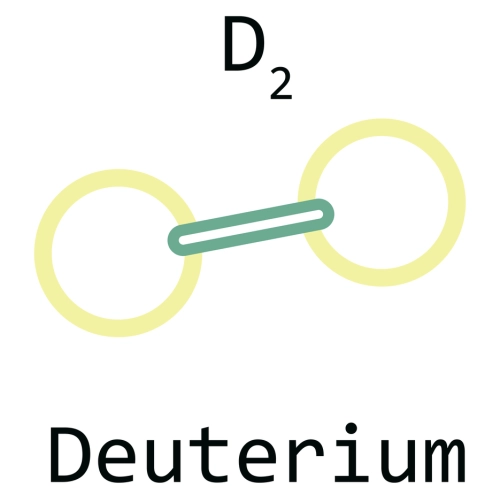 Deuterium is a stable isotope of hydrogen composed of one proton and one neutron. Its abundance in water is one atom for every 6,500 hydrogen atoms. This means that seawater has a concentration of 34 grams of deuterium per cubic meter of water.
Deuterium is a stable isotope of hydrogen composed of one proton and one neutron. Its abundance in water is one atom for every 6,500 hydrogen atoms. This means that seawater has a concentration of 34 grams of deuterium per cubic meter of water.
The energy content of deuterium is so high that the energy that can be obtained from the deuterium in one liter of seawater is equivalent to the energy that can be obtained from 250 liters of oil.
Considering the abundance of deuterium in the oceans, this energy source can be considered practically inexhaustible and sustainable.
2. Tritium
The other element used in nuclear fusion, tritium, is the unstable or radioactive isotope of the hydrogen atom. It is composed of one proton and two neutrons and decays relatively quickly by beta emission.
Although tritium is rare in nature, it can be generated by neutron capture reactions with lithium isotopes. Lithium is abundant in the Earth's crust and seawater.
Nuclear fusion research project
The most advanced project in nuclear fusion using magnetic confinement is ITER (International Thermonuclear Experimental Reactor), a prototype based on the Tokamak reactor concept, which is expected to achieve ignition.
Following the positive results achieved with the Joint European Torus (JET) , it was decided in 1990 to continue the fusion program with a larger facility where, in addition to the reactor, its auxiliary systems could be tested without yet generating electricity. The European Union, Canada, the United States, Japan, and Russia are participating in this project.
The objective is to determine the technical and economic feasibility of magnetic confinement nuclear fusion for generating electricity, as a preliminary phase to the construction of a commercial demonstration facility.
The ITER machine will not produce electrical energy; instead, it will test solutions to problems that need to be resolved to make future nuclear fusion reactors viable.