
The periodic table is a fundamental tool in the study of chemistry, which allows elements to be classified and organized according to their physical and chemical properties. Throughout history, the periodic table has evolved thanks to the contributions of scientists and chemists who have discovered new elements and refined its organization.
In this timeline of the periodic table, you will be able to discover the most important milestones in its development, from the first classifications of the elements to the inclusion of new synthetic elements in the 21st century.
Historical timeline
- 1789: The French chemist Antoine Lavoisier classifies the known elements into groups of oxides, acids, metals, and non-metals.
- 1829: German chemist Johann Wolfgang Döbereiner proposes the law of triads, which states that chemical elements can be grouped into triads in which the middle element has properties intermediate between the other two.
- 1864: British chemist John Newlands proposes the law of octaves, which shows the periodicity of elements in groups of eight.
- 1869: Russian chemist Dimitri Mendeleev publishes the first version of his periodic table, in which he organizes the elements by their atomic weight and shows the periodicity of chemical properties.
- 1871: The German chemist Lothar Meyer publishes his own version of the periodic table independently of Mendeleev's. Meyer relied heavily on the atomic weight of the elements. However, his contribution was not widely recognized in his time.
- 1913: British chemist Henry Moseley establishes that the atomic number of an element is the property that determines its position in the periodic table.
- 1914: German chemist Julius Lothar Meyer published a revised version of the periodic table, in which he organizes elements by their atomic number rather than their atomic weight.
- 1940: Glenn T. Seaborg and his colleagues contributed significantly to the discovery and synthesis of numerous transuranic elements (elements with atomic numbers greater than uranium). However, the expansion of the periodic table to include these elements was an ongoing process that spanned several decades.
- 1951: American physicist Edwin McMillan, in collaboration with Glenn T. Seaborg, contributes to the study of transuranic elements.
- 1955: Glenn T. Seaborg and his colleagues propose a revision of the periodic table to include the transuranic elements.
- 1962: British chemist Neil Bartlett discovers the first noble gas compound, expanding his understanding of noble gases and their position on the periodic table.
- 2016: The periodic table is expanded with the inclusion of four new elements, completing the seventh period of the table. These four elements are Nihonium (Nh), Moscovium (Mc), Tenesino (Ts), and Oganesón (Og).
Scientists linked to the periodic table
The development of the periodic table is the result of the work and contributions of various scientists over time.
Below are some of the most important scientists in the periodic table timeline:
Antoine Lavoisier (1743-1794)
 Lavoisier, known as the father of modern chemistry, contributed to the understanding of the conservation of mass and the formulation of the fundamental laws of chemistry.
Lavoisier, known as the father of modern chemistry, contributed to the understanding of the conservation of mass and the formulation of the fundamental laws of chemistry.
His research laid the foundations for the classification and systematic study of the elements.
Dmitri Mendeleev (1834-1907)
 Mendeleev, a Russian chemist, is considered one of the founding fathers of the modern periodic table. In 1869, he published his version of the periodic table, which organized the elements based on their chemical properties and their atomic masses. He also left empty spaces for elements that he predicted would yet be discovered, based on their properties.
Mendeleev, a Russian chemist, is considered one of the founding fathers of the modern periodic table. In 1869, he published his version of the periodic table, which organized the elements based on their chemical properties and their atomic masses. He also left empty spaces for elements that he predicted would yet be discovered, based on their properties.
Julius Lothar Meyer (1830-1895)
The German chemist Meyer also made important contributions to the organization of the periodic table around the same time as Mendeleev. Independently of Mendeleev, Meyer proposed a similar organization of elements based on their chemical properties.
Glenn T. Seaborg (1912-1999)
Seaborg was an American chemist who made significant contributions to the study of transuranic elements (elements beyond uranium on the periodic table) and to the discovery of several synthetic elements.
The element seaborgium (Sg) is named in his honor.
Conclusions
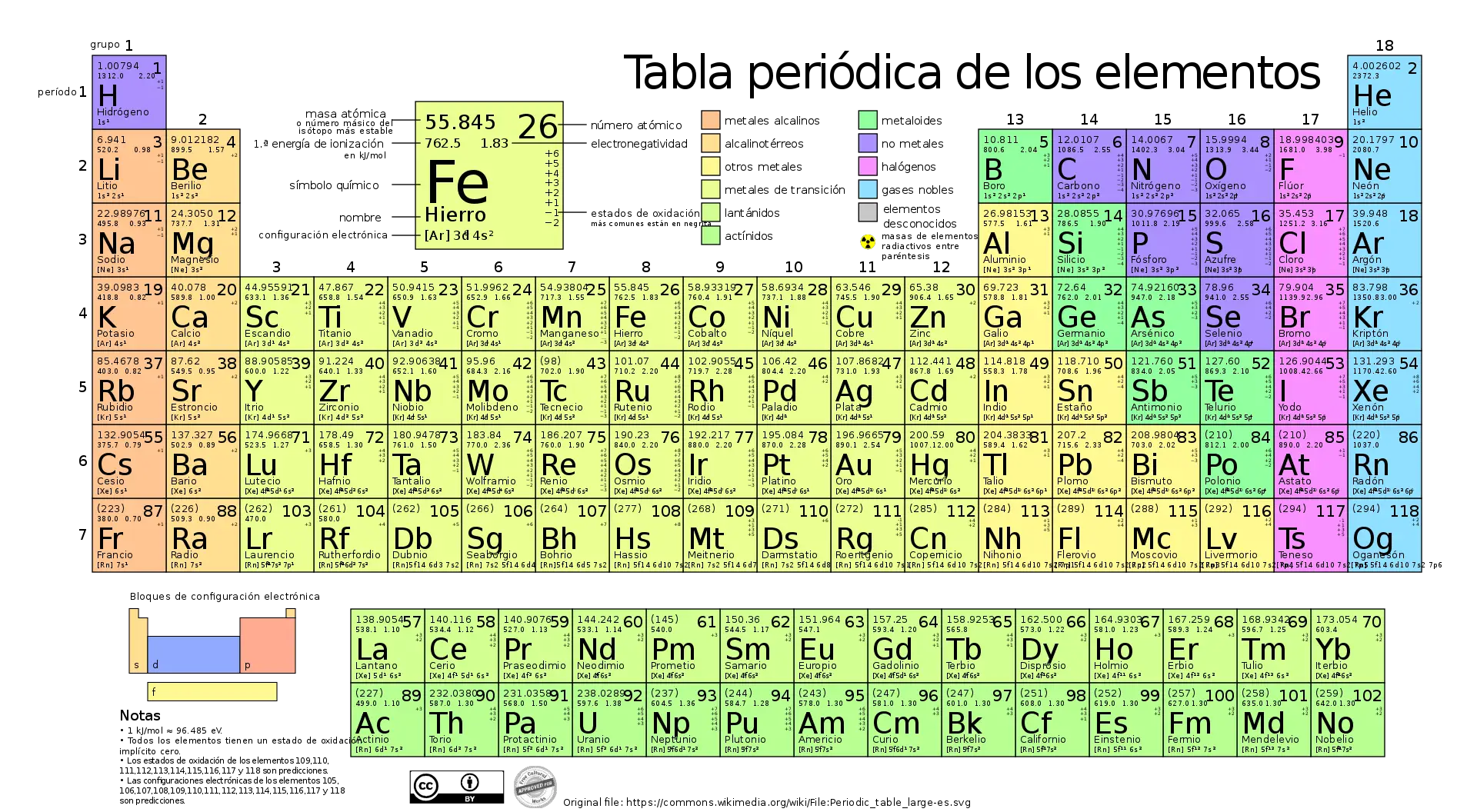 The timeline of the periodic table is a reflection of the evolution of chemistry over the centuries. From its beginnings in ancient times to its modern form based on quantum theory, the periodic table has been an invaluable tool for chemists and scientists around the world.
The timeline of the periodic table is a reflection of the evolution of chemistry over the centuries. From its beginnings in ancient times to its modern form based on quantum theory, the periodic table has been an invaluable tool for chemists and scientists around the world.
Its constant expansion and revision demonstrate that chemistry is a science in constant evolution, always seeking to better understand the world around us.