The density of water (H₂O) depends on many things, among them are the salt content in the water, the chemical elements and dissolved gases it contains, whether or not there is ice present, the temperature of the water or absolute relative humidity. .
The density of water is important because it determines how much a given volume of water weighs. The higher the density, the higher the weight.
The density of water is defined as the mass of water contained in a specific volume. In mathematical terms, it is expressed as:
Density = volume / mass
The standard unit for density in the international system is the kilogram per cubic meter (kg/m³), although the gram per cubic centimeter (g/cm³) is also used in certain contexts.
For water, the density is approximately 1000 kg/m³ at room temperature (20°C) and normal atmospheric pressure (1 atm). This means that one liter of water has a mass of approximately 1000 grams, or 1 kilogram.
Pure water density
The density of pure (distilled) water is 1 gram per cubic centimeter at 4 degrees Celsius, or what is the same: 1 kg/l. This means that there is one kilogram of water for every cubic decimeter (liter) of space it occupies.
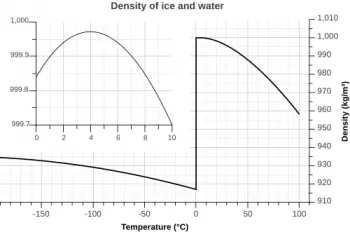
Water has its maximum density at 4 degrees Celsius. From this temperature, the water expands, increasing the temperature and also as it cools to zero degrees Celsius. At the freezing point of water (zero degrees Celsius), its density is 0.99980 kg/l. Once frozen, the density drops to the density of ice: 0.9168 kg/l.
Seawater density
 The density of sea water with a salinity index of 35 per thousand is 1.0267 kg/l.
The density of sea water with a salinity index of 35 per thousand is 1.0267 kg/l.
Ocean water is a mixture of water and mineral salts, so its density varies depending on the amount of mineral salts present and the temperature. In general, seawater has a higher density than freshwater due to the presence of these minerals.
As we have seen, when water is below four degrees Celsius, the density decreases. This property means that when water is close to its freezing point it is located in the surface water layers of oceans, lakes, etc. making aquatic life possible in the lower layers.
Table of densities of different types of water
The following table shows the density of different types of water at standard temperatures and pressures.
Density may vary slightly depending on specific conditions, but these values are approximate and refer to water under normal conditions:
|
Water type |
Density (kg/m³) |
|
Pure water (at 20°C) |
998.2 |
|
Seawater |
Approximately 1025 |
|
Distilled water |
1000 |
|
Saltwater |
Approximately 1020 |
|
Water at 0°C (ice) |
917 |
|
Water at 100°C (steam) |
0.6 (at 100°C and 1 atm) |
The density of seawater varies slightly depending on geographic location and the specific salinity of the water. The density of fresh water, such as distilled water, can also vary depending on purity and dissolved impurities. Finally, the density of ice is lower than that of liquid water due to the expansion it experiences when it freezes.
Factors that influence the density of water
Density depends mainly on two factors: temperature and pressure
Variation with temperature
An interesting characteristic of water is that its density varies with temperature. At lower temperatures, water is denser, while at higher temperatures, it becomes less dense. This has important implications for nature and everyday life.
At 4°C, water reaches its maximum density, which is approximately 1000 kg/m³. At this temperature, water molecules are organized more efficiently, resulting in higher density.
At lower temperatures, water molecules begin to form ice structures, expanding and decreasing their density. At higher temperatures, the molecules have more kinetic energy and move apart, which decreases the density of water.
Variation with pressure
 The density of water is also influenced by pressure. As the pressure increases, the density increases. This is particularly evident in the deep ocean, where the pressure is much higher than at the surface.
The density of water is also influenced by pressure. As the pressure increases, the density increases. This is particularly evident in the deep ocean, where the pressure is much higher than at the surface.
In the ocean, density increases as you go to greater depths. This is due to the compression of water molecules under the influence of pressure. The pressure at the bottom of the ocean can be thousands of times greater than the pressure at the surface, and this results in significantly higher density at depth.
The relationship between water density and pressure is important in oceanography and in understanding ocean circulation. The difference in density between water masses plays a crucial role in the formation of ocean currents and the mixing of nutrients in the oceans.
Relationship with specific gravity
The density of water is closely related to the specific gravity.
Specific gravity compares the density of a substance to the density of water at the same temperature and pressure conditions. The ratio is calculated by dividing the density of the substance by the density of water under those same conditions.
A specific gravity equal to 1 indicates that the substance has the same density as water, while values greater than 1 indicate a higher density and values less than 1, a lower density compared to water.
Specific gravity is used to compare the relative densities of different substances relative to water, which is valuable in a variety of scientific and engineering applications.
Importance of water density
Below are some of the reasons why water density is important:
Daily life
The density of water is essential for the buoyancy of objects and ships in water. Archimedes' principle is based on the difference in density between an object and water, which allows lighter objects to float and denser objects to sink.
Ecology
The variation in seawater density with depth and temperature affects the distribution of marine organisms.
Marine life is often found in specific layers of water that have a density suitable for their survival.
Science
In the field of oceanography, this property is crucial in the study of ocean circulation and the formation of water masses. Differences in density play a critical role in the formation of ocean currents and the mixing of nutrients in the oceans.
On the other hand, in meteorology, the density of water vapor is essential in cloud formation and precipitation. This variation in water vapor in the atmosphere influences climate and weather patterns.
Industry
Some examples of its importance in the industry are:
-
Nuclear power plants: in the nuclear industry it plays an important role in cooling nuclear reactors. The water acts as a coolant and moderator, transferring heat from the reactor core and slowing down neutrons to keep the nuclear reaction under control.
-
Food industry: Water density plays a crucial role in food formulation and production, especially in determining the concentration of solutions and the quality of products.
-
Oil and gas industry: In this sector, it is essential for the evaluation and control of fluids in drilling wells, guaranteeing safety in the exploration and production of oil and gas.
-
Process engineering and chemistry: In fields related to process engineering and chemistry, the density of water is widely used to calculate masses of substances and control chemical reactions in various industrial applications.
Ecology and conservation
In the field of ecology, it is important in the conservation of aquatic ecosystems, since it can affect the capacity of water bodies to support life and maintain ecological balance.