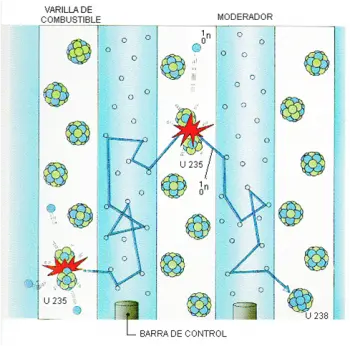
The neutron moderator plays a crucial role in nuclear reactors by participating in the management of nuclear fission reactions. Its main function lies in the task of reducing the speed of the neutrons generated during these reactions.
In the process of nuclear fission, neutrons collide with fissile uranium atoms, triggering additional reactions. Each fission event releases one or two neutrons at considerably high speeds, capable of starting a chain reaction by colliding with other surrounding atoms.
The key to efficiently harnessing nuclear energy lies in maintaining a controlled chain reaction within the reactor. If the neutrons maintain a high speed in the reactor core, the probability of them colliding with other atoms decreases.
In this context, the moderator assumes the responsibility of reducing the speed of the neutrons, thus improving the performance of the nuclear reactor. Neutrons released after an initial fission reaction travel at approximately 10% of the speed of light.
To ensure optimal operation of the reactor, it is essential to reduce this speed to a few kilometers per second, thus equating the conditions to temperatures of the order of a few hundred degrees Celsius.
Function of the nuclear moderator in the reactor
 Neutrons, driven by their high speed, harbor considerable kinetic energy. When a neutron collides with an atom of the material designated as the moderator, a fraction of its kinetic energy is transferred to the moderator atom, resulting in a simultaneous decrease in the speed of the neutrons.
Neutrons, driven by their high speed, harbor considerable kinetic energy. When a neutron collides with an atom of the material designated as the moderator, a fraction of its kinetic energy is transferred to the moderator atom, resulting in a simultaneous decrease in the speed of the neutrons.
The ideal chemical elements of the periodic table to play the role of moderator are those with a low atomic mass, thus maximizing the energy transferred in each collision. Among them, hydrogen, deuterium (present in heavy water) and carbon stand out.
The effectiveness of the moderator is based on its ability to reduce the speed of neutrons without absorbing them. To achieve this balance, it is imperative that moderator materials have a low effective trapping section.
However, at certain moments in the chain reaction, it is necessary to capture neutrons to maintain precise control. This is where the control rods come into play, acting strategically to regulate the amount of neutrons present and, therefore, manage the chain reaction.
Types of moderators
There are several types of nuclear moderators used in nuclear reactors to slow down neutrons and facilitate a controlled chain reaction. Here are some of the most common types:
- Light water (H₂O) : Plain water, composed primarily of hydrogen and oxygen atoms, is an effective moderator. In light water reactors, water acts as both a moderator and a coolant.
- Heavy water (D₂O) : Deuterium, an isotope of hydrogen, replaces hydrogen in heavy water. Heavy water reactors use this type of water as a moderator and coolant. Heavy water allows the use of unenriched natural uranium fuels.
- Graphite : Graphite, a form of carbon, is another common moderator. Graphite reactors use blocks of graphite to slow down neutrons. This material can also act as a reflector, redirecting some neutrons back to the nucleus.
- Light water and graphite (RBMK) : Some reactors, such as the RBMK type (Light Water and Graphite Reactors) used in the former Soviet Union, combine light water and graphite as moderators. This design allows for certain operational flexibilities.
- Organic compounds : Some reactors use organic compounds, such as hydrocarbons, as moderators. These materials offer efficient moderating properties and, in some cases, can simplify reactor design.
- Refractory Moderators : In certain high temperature reactors, refractory materials are used as moderators. These materials, such as beryllium oxide or silicon carbide, can withstand higher temperatures without losing effectiveness as moderators.