Atoms, the fundamental building blocks of matter, are made up of protons, neutrons and electrons. However, there are different versions of the same chemical element. These variants, known as isotopes, open the door to a world of atomic diversity and unique properties.
In this article, we'll explore some notable examples. From isotopes used in dating fossils and ancient rocks, to those that power us in nuclear reactors, we'll discover the characteristics and applications of some of these unique atomic elements.
Uranium isotopes are particularly relevant because they are used as fuel for nuclear power plants that use fission reactors. Deuterium and tritium, mentioned below, are the isotopes that are being worked with in nuclear fusion reactors.
Uranium and plutonium isotopes: Nuclear energy and weapons
Uranium-235 (²³⁵U)
Uranium-235 is the most important fissile isotope of uranium and one of the few materials capable of sustaining a chain reaction. Its ability to split into smaller nuclei when bombarded by neutrons makes it a key element for the generation of nuclear energy and the manufacture of nuclear weapons.
In nuclear reactors, enriched uranium-235 is used as fuel, generating large amounts of heat through controlled nuclear fission. This heat is used to heat water and produce steam, which in turn drives turbines that generate electricity. In addition, uranium-235 is used in the production of radioactive isotopes used in medicine, such as technetium-99m, which is used in gammagraphy for the diagnosis of diseases.
Because of its potential use in nuclear weapons, uranium-235 is subject to strict regulations and international control measures to prevent its proliferation.
Uranium-238 (²³⁸U)
It is the most abundant isotope of uranium, representing approximately 99.3% of natural uranium. Although it is not directly fissile, it can be transformed into plutonium-239 by neutron bombardment in nuclear reactors, making it a strategic material for the production of nuclear fuel.
In addition to its use in reactors, uranium-238 is used in radiometric dating of rocks and minerals using the uranium-lead method, allowing the age of geological formations to be determined. It is also used in the manufacture of high-density shielding for protection against radiation and in certain nuclear medicine treatments, such as positron emission tomography (PET).
Plutonium-239 (²³⁹Pu)
Plutonium-239 is a highly radioactive and fissile isotope used in nuclear weapons and advanced nuclear reactors. In atomic bombs, its ability to generate uncontrolled chain reactions has been exploited in highly destructive devices.
In the peaceful realm, plutonium-239 is used as an energy source in fission reactors and in radioisotope generators used in space probes, such as the Voyager and Curiosity missions, where its radioactive decay provides electrical power in environments where solar energy is not viable.
Hydrogen isotopes: Keys to nuclear fusion and science
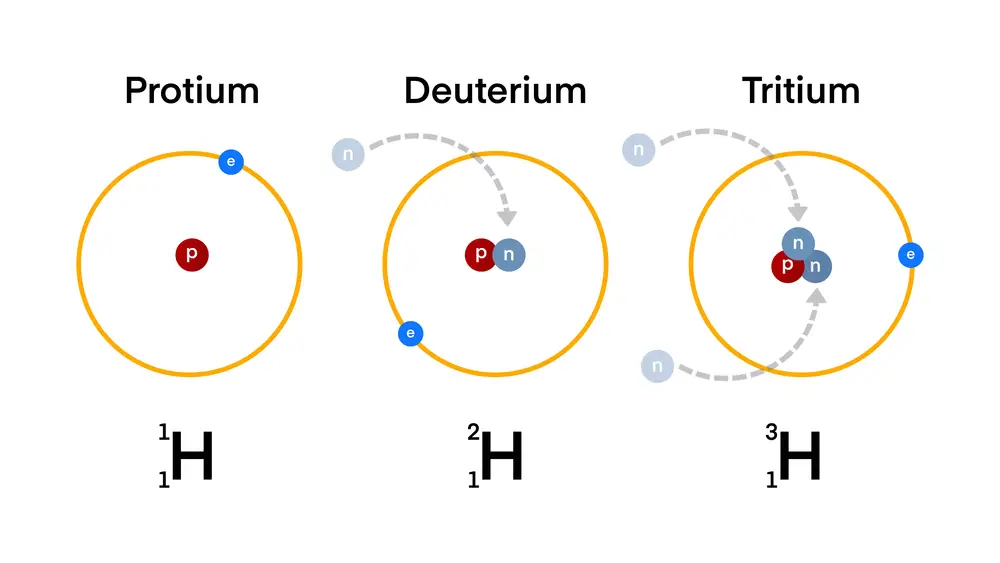
Hydrogen-2 (²H) or deuterium
Deuterium is a stable isotope of hydrogen with an extra neutron in its nucleus, making it heavier than ordinary hydrogen. Its most relevant application is in the production of heavy water (D₂O), used as a neutron moderator in certain types of nuclear reactors.
It also plays a crucial role in nuclear fusion research, where the possibility of combining deuterium and tritium to generate clean and sustainable energy is being studied. In addition, in chemistry and biomedicine, deuterium is used in nuclear magnetic resonance (NMR) studies to analyse the structure of molecules.
Hydrogen-3 (³H) or tritium
Tritium is a radioactive isotope of hydrogen that is used in experimental nuclear fusion reactors, where its combination with deuterium could provide an almost unlimited source of energy in the future. It is also used in radioluminescent lighting devices, such as emergency signals and clocks, due to its ability to emit light without the need for external energy.
In scientific research, tritium is used as a tracer in environmental and biological studies, allowing the analysis of water movement in ecosystems or metabolic processes in living organisms.
Hydrogen-1 (¹H) or protium
Protium is the most common isotope of hydrogen, accounting for approximately 99.98% of all hydrogen present on Earth. Its nucleus consists of a single proton with no neutrons, making it the lightest isotope of all the elements.
This isotope is essential in numerous natural and technological processes. In industry, it is used in the production of hydrogen gas (H₂), which is used as fuel in fuel cells to generate electricity cleanly. It is also key in the synthesis of ammonia using the Haber-Bosch process, which is essential for the production of fertilizers.
In the field of astrophysics, protium is the main component of the Sun and other stars, where it participates in nuclear fusion reactions that generate the energy that powers our solar system. It is also widely used in nuclear magnetic resonance (NMR) spectroscopy, a key technique for the structural analysis of molecules in chemistry and biology.
Isotopes used in dating and medical diagnosis
Carbon-14 (¹⁴C)
This radioactive isotope of carbon is essential in radiocarbon dating, a technique used to estimate the age of archaeological remains and fossils of organic origin. Its presence in living beings ceases when they die, and its disintegration allows us to calculate how much time has passed since then. This technique has been key in archaeology and paleontology, helping to understand the history of humanity and the planet.
Iodo-131 (¹³¹I)
Iodine-131 is a radioactive isotope used in nuclear medicine for the treatment of thyroid diseases, such as hyperthyroidism and thyroid cancer. Its beta radiation destroys abnormal thyroid cells without the need for invasive procedures, making it an effective therapeutic option. It is also used in thyroid function diagnostics using gammagraphy.
Tecnecio-99m (⁹⁹ᵐTc)
It is one of the most widely used isotopes in nuclear medicine due to its short half-life and its ability to emit gamma radiation without significantly affecting the patient. It is used in gammagraphy to visualize organs and tissues, helping in the detection of cardiovascular, bone and oncological diseases. Its use has revolutionized diagnostic imaging, allowing precise evaluations with minimal impact on the body.
Industrial and radiotherapy applications
Cobalt-60 (⁶⁰Co)
Cobalt-60 is a radioactive isotope with applications in cancer radiotherapy. Its powerful gamma ray emissions target cancer cells, damaging their DNA and preventing their proliferation. It is also used in the sterilization of medical equipment and in the irradiation of food to eliminate microorganisms without affecting its nutritional quality.