A proton is a subatomic particle with a positive electrical charge found within the atomic nucleus of atoms. The number of this sub-particle in the atomic nucleus determines the atomic number. The chemical elements are ranked in the periodic table of the elements accordingly to the atomic number.
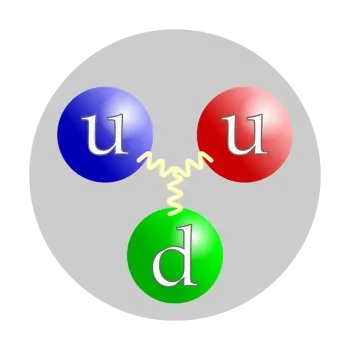
The proton is not an elementary particle but a compound particle. It is made up of three gluon-bound particles, two up quarks and one down quark. This structure means that it is a baryon (a subtype of particles called hadrons).
These atomic sub-particles are present in atomic nuclei, generally attached to neutrons by a strong nuclear force. Protons and neutrons are the smaller particles belonging to the nucleus of an atom, so they are also called nucleons. The only exception where it forms an atomic nucleus without any neutrons is the nucleus of an ordinary hydrogen atom - the most abundant nuclide in the universe.
However, hydrogen has other isotopes that do contain neutrons. For example, the nuclei of the heavy hydrogen isotopes (deuterium and tritium) contain a proton and one or two neutrons, respectively. These two hydrogen isotopes are used as nuclear fuel in nuclear fusion reactions. All other types of atoms are made up of two or more protons and different numbers of neutrons.
They may exist in:
-
Plasmas: they are the fourth state of aggregation of the matter. It is a fluid state similar to the gaseous state but in which a certain proportion of its particles are electrically charged (ionized) and do not possess an electromagnetic balance.
-
The cosmic rays, which are subatomic particles coming from space with a very high kinetic energy.
-
Solar wind: it is a stream of charged particles released from the Sun's upper atmosphere.
What is the life of a proton?
Its life is approximately 1035 years, so it is considered eternal at a practical level.
From the point of view of the standard model of particle physics, they are stable particles. The laws of physics do not allow a nucleon to decompose spontaneously due to preserving the number of baryons.
What is the mass of a proton?
It has a mass of about 1.6726219 × 10 -27 kilograms.
This value is roughly the same mass as neutrons. However, compared to the electron, the proton’s mass is about 1,836 times greater.
What is the electric charge of a proton?
The proton has a positive elemental charge of 1.602 x 10-19 coulombs.
Protons and electrons have the same absolute charge but with opposite signs. Therefore, neutral atoms have the number of electrons, and protons must be the same.
Why are protons necessary?
Protons are essential because they define what element an atom is.
The atomic number (Z) is the number of protons in its nucleus and determines the atom’s chemical properties.
The number of neutrons (N) is also used by adding all the nucleons to determine the isotopes of an element. Hence, the mass number (A) is the sum of both sub-particles.
Another essential characteristic is that its positive charge helps capture electrons and keep them orbiting around the atom’s nucleus.
Who discovered the proton?
The proton was discovered by Ernest Rutherford in 1919.

The history of its discovery dates back to 1886 when Eugene Goldstein discovered anodic rays and showed that they were positively charged particles (ions) produced from gases.
With his experiments, Goldstein observed that these particles had different values of the relationship between charge and mass. For this reason, the positive charge with a particle could not be identified.
In 1911, Ernest Rutherford presented his planetary atomic model. In this model, he already established that a positive electric charge was concentrated in the center of the atom, surrounded by electrons discovered by Thompson with a negative charge.
Rutherford freed the hydrogen nucleus using a source of radioactivity to produce energetic alpha particles. In addition, Rutherford showed that the hydrogen nucleus was present in other nuclei with his research.
Hydrogen is the only element in the periodic table with a single proton. Therefore, when referring to a hydrogen nucleus, it was like talking about a proton.