
Radioactivity, an undeniably powerful phenomenon in the world of nuclear physics, plays a critical role in the nuclear energy industry.
Nuclear energy has long been a source of interest and debate, due to its ability to provide a highly concentrated and relatively clean source of energy. However, this energy source carries considerable risks, which largely arise from the nature of the radioactivity itself.
In this article, we will carefully explain the concept of radioactivity, its importance in the generation of nuclear energy and the implications in terms of safety that this entails.
What is radioactivity?
Radioactivity is a property that some chemical elements possess due to the instability of their atomic nuclei. These elements are called "radioisotopes" and they spontaneously emit subatomic particles or electromagnetic radiation in a process known as radioactive decay or radioactive decay.
Radioactive decay occurs in unstable atomic nuclei. That is, those that do not have enough binding energy to hold the nucleus together.
Types of radioactivity: Alpha, Beta and Gamma
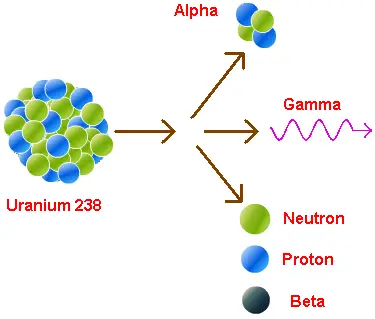
Alpha (α) radiation
Alpha particles consist of helium nuclei, composed of two protons and two neutrons.
These particles are relatively large and heavy compared to other forms of radiation, making them poorly penetrating. In fact, a simple sheet of paper or even human epidermis can effectively stop alpha particles.
However, if inhaled or ingested, they can be dangerous as they can damage cells in direct contact with internal tissues.
Beta (β) radiation
Beta radiation involves high-energy particles: electrons (β-) or positrons (β+). These particles are smaller and lighter than alpha particles, and have a greater penetration capacity.
Beta particles can pass through skin and tissues, but are blocked by materials such as glass or plastic. Beta radiation can also be dangerous if radioactive material is swallowed or inhaled.
Gamma radiation (γ)
Gamma radiation comes in the form of highly energetic electromagnetic rays, similar to X-rays, but with higher energy.
Gamma rays are highly penetrating and can pass through dense materials such as lead or concrete. Due to its high energy and penetrating ability, gamma radiation is especially dangerous to humans and requires appropriate protective measures in exposure environments.
Origin of radioactivity: natural and artificial
Radioactivity can have a natural or artificial origin:
Natural radioactivity
Natural radioactivity is inherent to the Earth and our environment; it occurs in nature due to chains of natural radioactive elements of non-anthropogenic origin.
Some elements, such as uranium, thorium, and radium, have unstable isotopes that decay over time, emitting radiation. Natural background radiation is the constant, low exposure to this radiation that we all experience in everyday life.
Radiation of natural origin was discovered by chance by Antoine-Henri Becquerel. Later, with Becquerel's experiments, Marie Curie discovered other radioactive substances.
Artificial radioactivity
 Artificial radioactivity is any radioactivity or ionizing radiation of human origin. It involves the creation of radioisotopes through nuclear bombardment or irradiation processes. These radioisotopes are used in various applications, such as medicine and nuclear power generation.
Artificial radioactivity is any radioactivity or ionizing radiation of human origin. It involves the creation of radioisotopes through nuclear bombardment or irradiation processes. These radioisotopes are used in various applications, such as medicine and nuclear power generation.
An example of artificial radioactivity is that generated in nuclear medicine or in the nuclear fission reactions of nuclear power plants to obtain electrical energy.
Applications in science
 Radioactivity has a wide range of beneficial applications in different fields:
Radioactivity has a wide range of beneficial applications in different fields:
-
Medicine: nuclear medicine uses radioisotopes for diagnosis (positron emission tomography - PET) and treatment (radiotherapy) of diseases such as cancer.
-
Radiometric dating: Radiometric dating is used in geology and archeology to determine the age of objects and rocks, based on the amount of radioisotopes present.
-
Non-destructive inspection and testing: Industrial radiography uses x-rays or gamma radiation to inspect materials without physically damaging them, essential in industry and construction.
-
Power generation: Nuclear energy provides a source of clean electricity, although with challenges in waste management and safety.
Hazards to human health and the environment
Radioactivity, despite its usefulness in various applications, poses significant risks to both human health and the environment.
Human health risks
Cell damage and cancer risk
Exposure to ionizing radiation, such as that emitted from radioactive materials, can damage cells and DNA in the human body. In the long term, this can increase the risk of developing cancer.
The degree of risk depends on several factors, including the radiation dose and duration of exposure. Alpha, beta and gamma radiation can have harmful effects on human health if not properly controlled.
Acute and chronic effects
In addition to the risk of cancer, acute exposure to high doses of radiation can cause immediate effects such as tissue and organ damage, acute irradiation syndrome, and, in extreme cases, death as happened to Hisashi Ouchi in the Tokaimura accident.
On the other hand, chronic exposure to low doses of radiation can cause non-immediate effects, such as cardiovascular disease or cataracts.
Environmental risks
Radioactive pollution
 The uncontrolled release of radioactive materials into the environment can result in radioactive contamination.
The uncontrolled release of radioactive materials into the environment can result in radioactive contamination.
This can occur in nuclear disasters, industrial accidents or during improper management of radioactive waste. Two examples that illustrate this danger are the nuclear accidents at Chernobyl and Fukushima.
Radioactive contamination can affect wildlife, aquatic and terrestrial ecosystems, and the food chain, with long-term impacts on biodiversity and environmental health.
Radioactive waste
Nuclear power generation and other radioactive applications produce radioactive waste, some of which has an extremely long half-life.
The safe management of this waste is a major challenge, as it must be stored safely for thousands of years to avoid contamination of the environment and exposure to radiation.