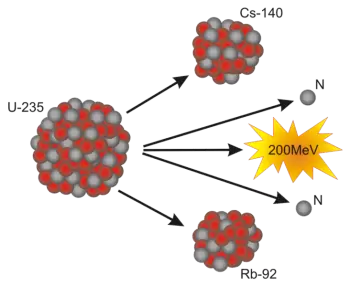
Nuclear fission is a nuclear reaction in which the nucleus of a heavy atom splits into two or more smaller fragments, releasing a large amount of energy.
This reaction can be induced by the capture of a neutron by a nucleus, or spontaneously due to the instability of the isotope.
Atomic nuclei are composed of protons and neutrons. Protons have a positive charge and tend to repel each other, while neutrons have no charge. These particles are held together by the strong nuclear force, which is much stronger than the electromagnetic force that keeps electrons around the nucleus.
Nuclear fission involves breaking this nuclear force, allowing nucleons (protons and neutrons) to separate.
How does nuclear fission work?
Nuclear fission is a process in which the nucleus of a heavy atom, such as uranium-235 or plutonium-239, splits into two or more lighter nuclei, releasing a large amount of energy.
This process begins when a free neutron hits the nucleus of a fissile atom, causing the nucleus to become unstable and break apart. As the nucleus splits, it releases additional neutrons, which can continue to hit other nuclei, thus starting a chain reaction.
The energy released in fission comes from the loss of mass during the splitting of the nucleus, which is converted into energy according to Einstein's famous equation, \(E = mc^2\). The fragments resulting from fission are fission products, which are different chemical elements, and the released neutrons can be used to continue the process.
In nuclear reactors, this controlled reaction generates heat that is converted into electrical energy. However, in nuclear weapons, fission occurs uncontrolled, releasing an enormous burst of energy.
Energy generation in nuclear fission
The energy released in nuclear fission comes from the conversion of a small amount of mass into energy, according to Einstein's equation, \( E = mc^2 \), where:
- E is the energy obtained,
- m is the mass "lost" during fission,
- c is the speed of light in vacuum (299,792,458 m/s).
This energy is released in the form of heat, which can be used in nuclear reactors to generate electricity.
The chemical element in nuclear fission
The fissile material used in nuclear reactions is usually uranium-235 (U-235) or plutonium-239 (Pu-239).
These isotopes are unstable due to the large number of protons in their nuclei, which makes the atomic structure prone to fission when it interacts with a neutron. Isotopes are versions of the same element with different numbers of neutrons, and fission usually occurs when a neutron hits a uranium-235 or plutonium-239 atom, triggering a chain of reactions.
Chain reactions
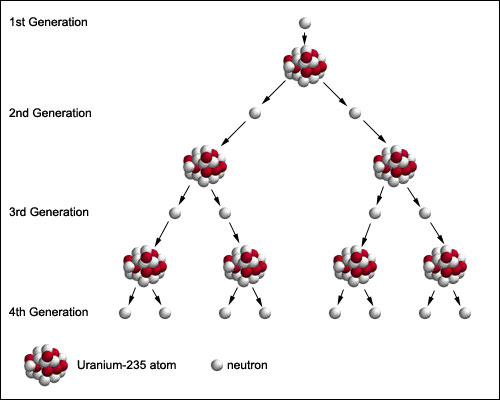 Nuclear fission can produce a chain reaction.
Nuclear fission can produce a chain reaction.
In this process, neutrons released by one fission induce further fissions in other atoms, generating more neutrons and releasing more energy. The resulting neutrons can be absorbed by other fuel atoms, keeping the reaction going. This chain can be controlled or uncontrolled:
- Controlled : In nuclear reactors, where the amount of neutrons is regulated by control rods (made of materials such as boron or cadmium that absorb neutrons), allowing the reaction to be maintained at a constant and safe level.
- Uncontrolled : In a nuclear explosion, such as in atomic bombs, where the reaction accelerates out of control and an enormous amount of energy is released quickly and destructively.
Critical mass and reaction control
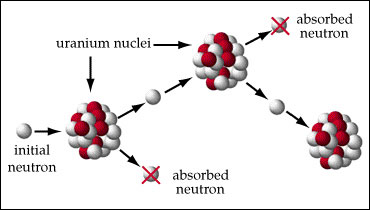 Critical mass is the minimum amount of fissile material that is necessary for the chain reaction to be self-sustaining.
Critical mass is the minimum amount of fissile material that is necessary for the chain reaction to be self-sustaining.
If the fissile material is less than the critical mass, neutrons are lost faster than they are generated, and the reaction stops. The amount of critical mass depends on factors such as the geometry of the material, its purity, and its physical properties.
Spontaneous nuclear fission
In spontaneous nuclear fission , an atom can split without the need for a neutron to hit it. This phenomenon occurs naturally in some isotopes, such as plutonium-239, which has a higher spontaneous fission rate than uranium-235. Although this process is rare, it can contribute to the radioactive activity of certain materials.
Applications of nuclear fission
Nuclear fission has various applications :
- Electricity generation : In nuclear power plants, the energy released in fission is used to heat water and produce steam, which drives electricity-generating turbines.
- Nuclear Submarine Propulsion : Nuclear submarines use fission reactors to generate power and allow the submarines to operate for long periods without needing to refuel.
- Atomic bombs : Nuclear fission is also used in nuclear weapons, where runaway reactions cause a massive release of energy.
- Plutonium production : Through irradiation of uranium-238, plutonium-239 can be produced in nuclear reactors, a key process in the manufacture of nuclear fuels and nuclear weapons.
Fusion vs. Fission
It is important not to confuse nuclear fusion with nuclear fission . Fusion involves the joining of two light nuclei to form a heavier one, releasing energy in the process, as occurs in the Sun. Fission, in contrast, involves the splitting of a heavy nucleus into smaller fragments, releasing energy. Nuclear fusion still faces significant technical challenges for its practical use in power generation, while fission is widely used today.
In summary, nuclear fission is a powerful and complex reaction that not only provides us with a significant source of energy, but also poses significant challenges in terms of safety and radioactive waste management.