Molar mass is a physical property used to describe the average mass of an atom or molecule of a substance in units of grams per mole (g/mol) and is represented by the letter "M."
In other words, molar mass indicates how many grams of a substance are in one mole of that substance. The mole is a unit of quantity of substance in the International System of Units (SI).
Measurement units
In the International System of Units (SI), the unit for measuring molar mass is kilogram per mole (kg/mol). However, when the molar mass is expressed in g/mol, its numerical value is the same with the relative molecular weight. For this reason, historically molar weight is generally expressed in grams per mole (g/mol).
Molar mass in formulas is usually indicated by a capital letter M.
Direct comparisons and measurements of the masses of atoms and molecules are made using mass spectrometry methods.
Molar mass calculation
Calculating the molar mass of a compound is done by adding the masses of all the atoms in a molecule using the relative atomic masses.
Relative atomic masses are numbers that represent the mass of an atom relative to the mass of the carbon-12 atom, which is defined as 12 atomic mass units (amu).
For example, the relative atomic mass of hydrogen is approximately 1 amu, while the relative atomic mass of oxygen is approximately 16 amu.
Complex molecules
 To calculate the number of molar masses of complex molecules, it can be determined by adding the molar masses of their constituent elements. For example, the molar mass of water H 2 O is
To calculate the number of molar masses of complex molecules, it can be determined by adding the molar masses of their constituent elements. For example, the molar mass of water H 2 O is
M(H 2 O) = 2 M(H) + M(O) = 2.1 g/mol + 16 g/mol = 18.1 g/mol
How to calculate molar mass step by step?
Calculating the molar mass of a chemical substance can be done by following these steps:
-
Identify the chemical formula of the chemical compound.
-
Determine how many atoms of each element there are in the chemical formula.
-
Multiply the number of atoms of each element by its relative atomic mass.
-
Add the masses of all the atoms in the formula to obtain the molar mass.
Practical example
Let's look at a concrete example to calculate the molar mass of water (H₂O):
-
The chemical formula of water is H₂O, which means it contains two hydrogen atoms (H) and one oxygen atom (O).
-
The relative atomic mass of hydrogen is approximately 1 amu and that of oxygen is approximately 16 amu.
-
To calculate the molar mass of water, we add the masses of the atoms: (2 * 1 amu) + (1 * 16 amu) = 2 amu + 16 amu = 18 amu.
Therefore, the molar mass of water is 18 g/mol.
Examples
Below is a table with examples of some molar masses of different substances:
|
substance |
Chemical formula |
Molar mass (g/mol) |
Description |
|
Hydrogen |
H₂ |
2 |
Hydrogen is the lightest element, with a molar mass of 2 g/mol. |
|
Oxygen |
O₂ |
32 |
Oxygen is essential for respiration and has a molar mass of 32 g/mol. |
|
Carbon dioxide |
CO₂ |
44 |
CO₂ is a greenhouse gas with a molar mass of 44 g/mol. |
|
Ammonia |
NH₃ |
17 |
Ammonia is used in the chemical industry and has a molar mass of 17 g/mol. |
|
Methane |
CH₄ |
16 |
Methane is the main component of natural gas and has a molar mass of 16 g/mol. |
|
Sulfuric acid |
H₂SO₄ |
98 |
Sulfuric acid is a widely used strong acid with a molar mass of 98 g/mol. |
|
Glucose |
C₆H₁₂O₆ |
180 |
Glucose is a sugar found in foods and has a molar mass of 180 g/mol. |
|
Sodium Chloride (table salt) |
NaCl |
58.5 |
Sodium chloride is common table salt and has a molar mass of 58.5 g/mol. |
|
Uranium |
OR |
238.03 |
Uranium is an element used as fuel in nuclear reactors. |
|
Plutonium |
Pu |
244 |
Plutonium is an element used in nuclear weapons and nuclear reactors. |
|
Heavy Water |
D₂O |
20.03 |
Heavy water contains deuterium instead of hydrogen and is used in nuclear reactors. |
|
Enriched uranium |
U-235 |
It varies |
Enriched uranium contains a higher proportion of U-235 and is essential for nuclear fission. |
What is molar mass used for?
Molar mass is a fundamental property that has several implications and applications in chemistry and other scientific disciplines.
Some of its most notable applications include:
-
Conversion between moles and grams: used to convert between the amount of substance in moles and the mass in grams of a substance.
-
Determination of the composition of substances.
-
Calculation of chemical reactions: It is essential for calculating the quantities of reactants and products in a chemical reaction, allowing for stoichiometry and experiment planning.
-
Identification of unknown substances: Used in analytical techniques, such as mass spectroscopy, to identify unknown substances through comparison of their experimental molar masses with calculated molar masses.
-
Applications in thermodynamics: in chemical thermodynamics it is used to calculate properties such as enthalpy and entropy of chemical reactions.
Importance of everyday life
Molar mass is not only important in the laboratory, but also has applications in everyday life. Some examples include:
-
Nutrition : In the food industry, it is used to calculate the amount of nutrients in foods and to establish daily consumption recommendations for essential nutrients such as proteins, carbohydrates and fats.
-
Pharmacology : In drug formulation, it is crucial to know the molar mass of the active components and excipients to ensure proper dosage and efficacy of the drug.
-
Energy : In the energy industry, molar mass is used in the production and use of fuels and renewable energy, such as calculating fuel density and fuel cell efficiency.
Relationship with the periodic table
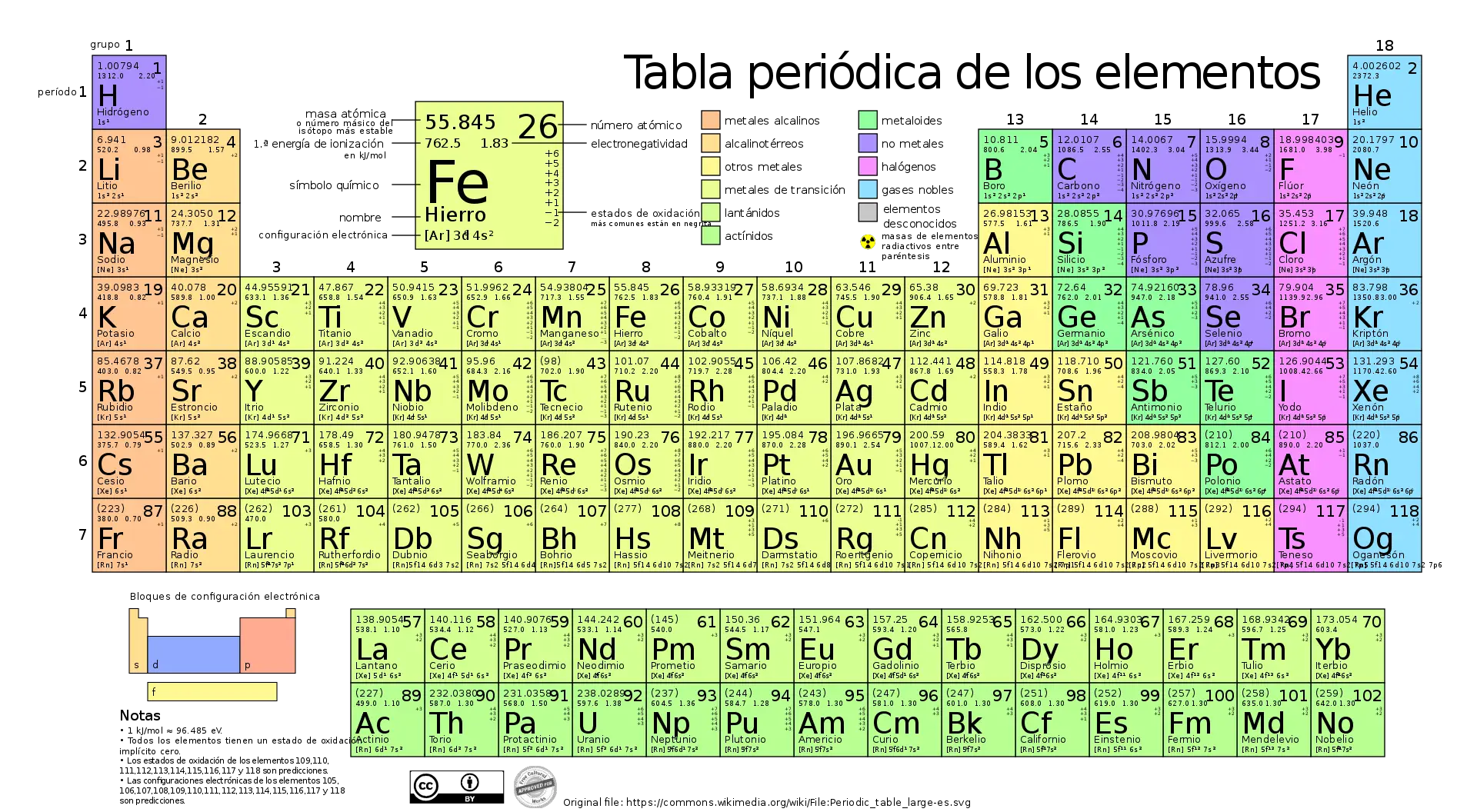 The periodic table of chemical elements is an invaluable tool for determining the molar masses of elements and compounds. Each element in the periodic table has a relative atomic mass that is rounded to whole numbers or simple fractions in the case of isotopes.
The periodic table of chemical elements is an invaluable tool for determining the molar masses of elements and compounds. Each element in the periodic table has a relative atomic mass that is rounded to whole numbers or simple fractions in the case of isotopes.
To calculate the molar mass of a compound, simply add the relative atomic masses of the elements present, multiplied by the number of atoms of each element in the formula.
Example of sodium chloride
An interesting example is calculating the molar mass of sodium chloride (NaCl).
Sodium (Na) has a relative atomic mass of about 23 amu, and chlorine (Cl) has a relative atomic mass of about 35.5 amu.
Therefore, the molar mass of NaCl is the sum of these atomic masses: 23 amu + 35.5 amu = 58.5 amu, which is equivalent to 58.5 g/mol.
Not to be confused with...
Molar mass is often confused with other concepts in chemistry and physics because they all relate to the weight or amount of a substance. Here are some concepts that it is commonly confused with:
- Molecular mass : Molecular mass is the sum of the atomic masses of the atoms in a molecule, the same as molar mass but expressed in atomic mass units (amu or uO) , not g/mol. Molar mass and molecular mass have the same numerical value, but differ in their units and the context in which they are used.
- Atomic mass : This is the average of the mass of the atoms of an element, taking into account the abundance of its isotopes, and is measured in atomic mass units (u). Atomic mass is used to calculate the molar mass of an element in the context of a substance.
- Molecular weight : This term was formerly used as a synonym for molecular mass, but this is not correct. Weight is a force (resulting from gravity acting on a mass) and is measured in newtons (N), whereas molecular mass is a measure of the amount of matter in atomic mass units. It is more correct to speak of molecular mass than molecular weight.
- Amount of substance (mole) : Sometimes confused with molar mass because both concepts involve the mole. However, amount of substance (mole) is a measure of the number of particles (atoms, molecules, ions) in a sample and not their mass. Molar mass, on the other hand, represents the mass of one mole of those particles.
- Molar volume : This concept refers to the volume occupied by one mole of a substance, especially in the context of gases, and is usually expressed in L/mol. It is different from molar mass, which measures the amount of mass in grams per mole of substance.