
The atom is a structure in which matter is organized in the physical world or in nature. Its structure is made up of different combinations of three sub-particles: neutrons, protons, and electrons. Molecules are made up of atoms grouped by chemical bonds.
It is the smallest unit of matter of which an element can be constituted.
This is an example in order to understand this concept better: We have a piece of iron which we split. We still have two smaller pieces of iron. Afterward, we keep splitting those pieces... We will have more and more smaller pieces.
There will come a time when we will only have a piece so small that it can no longer be broken. If we could break it, it would no longer be iron, it would be another element of the periodic table. This very small piece is an iron atom.
Atom definition in chemistry
We define an atom as the smallest particle into which an element can be divided without losing its chemical properties.
The origin of the word comes from the Greek, which means indivisible. At the time these particles were baptized, it was believed that they could not be divided effectively, although today we know that they are made up of even smaller particles.
Structure and parts of the atom
The atom is made up of three sub-particles:
-
Protons, positively charged.
-
Neutrons, without electrical charge (electrically neutral).
-
Electrons, which are negatively charged.
In turn, it is divided into two parts:
-
The nucleus. Formed by neutrons and protons in the center of the atom. These two particles are called nucleons because of that.
-
The crust, which is formed only by electrons.
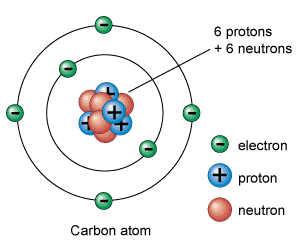
Protons, neutrons, and electrons are the subatomic particles that make up the atomic structure. What differentiates them between them is the relationship established between them.
Electrons are the lightest subatomic charged particles. Positively charged protons weigh about 1,836 times more than electrons. Neutrons, the only ones without an electrical charge, weigh about the same as protons.
Protons and neutrons are grouped together in the atomic nucleus. This is the reason which they are also called nucleons. The energy that holds neutrons and protons together is nuclear energy.
Therefore, the atomic nucleus has a positive charge (that of protons). Almost all of the atom mass is concentrated in the nucleus.
On the other hand, around the nucleus, there are a certain number of negatively charged electrons. The total (positive) charge of the nucleus is equal to the negative charge of the electrons, so that the total electric charge is neutral. It means that if the atom is not ionized, the number of electrons must be the same as the number protons.
Size of the atom
Atoms are not delimited by a clear boundary, so their size equals that of their electronic cloud.
However, a measurement of this cannot be established either, due to the wave properties of the electrons. In practice, the atomic radius is defined by estimating it based on some physical phenomenon, such as the quantity and density of atoms in a given volume, or the distance between two nuclei in a molecule.
Energy levels
An electron bound in the atom has a potential energy inversely proportional to its distance from the nucleus and of negative sign. It means that it increases with distance.
The magnitude of this energy is the amount necessary to detach it, and the unit commonly used to express it is the electron volt (eV).
Atomic theory
Today the idea that matter is composed in this way is well established scientifically.
However, throughout history, different theories on the composition of matter have been developed. They are the atomic models.
These are some of the theories and models defined throughout the history of nuclear energy.
-
John Dalton's atomic theory. He imagined atoms as tiny spheres.
-
The atomic model of Niels Bohr.
-
Ernest Rutherford’s model
-
Thomson's atomic model.
The description of the electrons orbiting the nucleus corresponds to the simple Niels Bohr model.
Atom characteristics and properties
The basic units of chemistry are atoms. During chemical reactions they remain as such, they are neither created nor destroyed. They are simply organized differently by creating different links between them.
 Atoms are grouped together forming molecules and other types of materials.
Atoms are grouped together forming molecules and other types of materials.
According to the composition, the different chemical elements represented in the periodic table of the chemical elements are differentiated. In the periodic table we can find the atomic number and the mass number of each element:
-
Atomic number, number of protons.
-
Mass number, the sum of protons and neutrons.
Atomic number
It is represented by the letter Z.
This number indicates the number of protons in the nucleus.
The number of protons determines in which element an atom belongs.
For example, if they have a single proton, it is a hydrogen atom (Z = 1).
Mass number
The mass number is represented by the letter A.
It refers to the sum of protons and neutrons that the element contains. Isotopes are two atoms with the same number of protons, but different numbers of neutrons.
Isotopes of the same element have very similar chemical and physical properties.
Atomic weight is the ratio of the average mass of a chemical element’s atoms to some standard.
What are isotopes?
It happens that the atoms of an element do not all have the same number of neutrons in the nucleus. This is called an isotope. Isotopes have (almost) the same chemical properties, but other physical properties. More than one isotope of virtually all elements is known.
Isotopes are very important in the nuclear power industry. Uranium enrichment is about converting one uranium isotope into another, more unstable uranium isotope. Without these highly unstable isotopes, fission chain reactions could not be generated.
Relationship of atoms to molecules
A molecule is formed when two or more atoms are joined by chemical bonds. Atoms can be joined together by bonds. These bonds are formed when atoms share, gain, or lose electrons to achieve a more stable electronic configuration.
In a molecule, the atoms are arranged in a three-dimensional structure and are held together by chemical bonds. The way in which the atoms are joined in a molecule determines its chemical and physical properties, as well as its behavior in chemical reactions.
Where does the energy of atoms come from?
The energy of atoms comes mainly from two sources:
- the nuclear energy
- electronic energy.
Nuclear energy
Nuclear energy is related to the forces that hold atomic nuclei together. Nuclear energy is released or absorbed during nuclear processes, such as nuclear fission and nuclear fusion. In nuclear fission, a heavy nucleus is split into smaller nuclei, and in nuclear fusion, two light nuclei join to form a heavier one. Both processes release energy due to differences in the masses of the nuclei involved, according to Einstein's famous equation, E = mc².
Electronic energy
For its part, electronic energy is associated with the configuration of electrons in atoms. Electrons orbit around the atomic nucleus in energy levels or electron shells.
Electrons can move between these energy levels by absorbing or emitting energy in the form of photons. When electrons absorb energy, they can jump to higher energy levels. When they release energy, they return to lower energy levels.
These changes in electronic energy are responsible for the emission and absorption of light, as well as other forms of electromagnetic radiation, such as X-rays.