
Uranium, a chemical element of great importance in the world of science and technology, occupies a prominent place in the periodic table due to its unique properties. Its atomic number is 92, and it is found in nature in various forms.
Uranium is best known for its role in nuclear power generation and nuclear weapons manufacturing, as it is the key element in nuclear fission. In addition, it has applications in medicine, scientific research and aerospace technology.
Although its use offers significant benefits, uranium is radioactive and its handling is subject to strict regulations to ensure its safe use.
Uses and applications
 Uranium has several applications in industry and research. Let's look at some examples:
Uranium has several applications in industry and research. Let's look at some examples:
- Nuclear energy generation: The most prominent use of uranium is as fuel in nuclear reactors for the generation of electrical energy. Enriched uranium is used in nuclear fission, where uranium-235 nuclei split into smaller nuclei, releasing a large amount of energy as heat. This energy is converted into electricity. Nuclear energy is a clean and efficient energy source.
- Manufacturing of nuclear weapons: Enriched uranium has also been used in the manufacture of nuclear weapons. When a sufficient concentration of uranium-235 is reached, it can be used to create devastating nuclear explosions. This has led to the need to control and restrict access to enriched uranium.
- Nuclear medicine: Uranium is used in nuclear medicine in the production of radioisotopes, such as technetium-99m, which are used in medical imaging, such as positron emission tomography (PET) and scintigraphy, for the diagnosis of diseases and disorders.
- Space propulsion: Nuclear fission of uranium has been considered as an energy source for long-duration space missions. The possibility of using nuclear reactors to power spacecraft has been explored.
- Scientific research: Uranium is used in scientific research, including nuclear experiments and studies on particle physics. It is also used in particle accelerators and radiation detectors.
- Armor-piercing and armor-piercing projectiles: Depleted uranium has been used in armor-piercing projectiles and the armor of military vehicles due to its high density, which provides it with penetrating and protective properties.
Structure of a uranium atom
 An atom consists of a nucleus and electrons that surround this nucleus. In turn, a nucleus consists of protons and neutrons. A proton has a positive charge. A neutron has no electrical charge and is neutral.
An atom consists of a nucleus and electrons that surround this nucleus. In turn, a nucleus consists of protons and neutrons. A proton has a positive charge. A neutron has no electrical charge and is neutral.
The positive charges of the protons try to violently push each other out. What prevents them from separating is a new kind of force: an immensely powerful, short-range force of attraction that acts equally between protons and neutrons (which, from this point of view, are all nucleons).
The short-range nuclear force keeps them together, opposing the repulsive effect of the positive charges of the protons. In this way, neutrons act as “nuclear cement.”
Types of uranium: natural, enriched and depleted
There are different types of uranium depending on its isotopic composition. Each of these types has specific properties and applications, making them vital components of nuclear technology and other related disciplines.
Below is a brief description of each type of uranium and their respective characteristics.
natural uranium
Natural uranium is that found in nature and is mainly composed of three isotopes: uranium-238 (U-238), uranium-235 (U-235) and uranium-234 (U-234). The proportion of U-238 in natural uranium is much higher than that of U-235, and it needs to be enriched for use in nuclear reactors or nuclear weapons.
Enriched uranium
 Enriched uranium is one in which the proportion of U-235 is increased compared to natural uranium. This process is carried out in enrichment plants and is used to obtain uranium with a higher concentration of U-235. Enriched uranium is needed to fuel most nuclear reactors used for power generation.
Enriched uranium is one in which the proportion of U-235 is increased compared to natural uranium. This process is carried out in enrichment plants and is used to obtain uranium with a higher concentration of U-235. Enriched uranium is needed to fuel most nuclear reactors used for power generation.
Depleted uranium
Depleted uranium is uranium that has gone through the enrichment process to increase the concentration of U-235, and the remaining material, which has a lower concentration of U-235, is known as depleted uranium. This material has limited uses and is often stored or used in non-nuclear applications.
Uranium on the periodic table
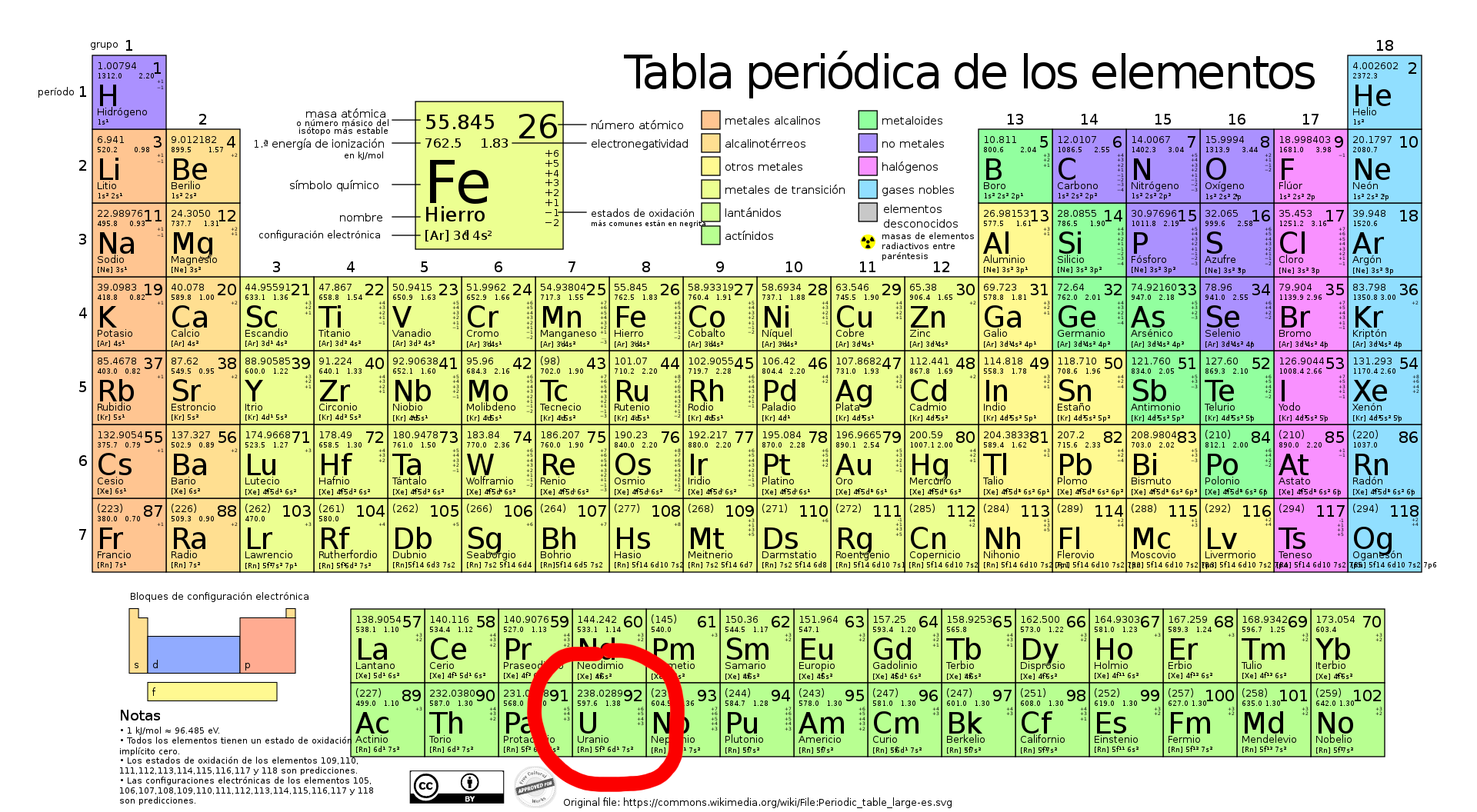
Uranium, with symbol "U" and atomic number 92 on the periodic table, is a radioactive element with several notable isotopes found in group 3 of the periodic table.
It belongs to period 7, which means that its seventh energy level contains electrons. Regarding its block, it is classified as an f-block element, specifically in the actinide series.
This element is part of the actinide series due to the similarity in electronic properties of the elements within this group.
|
Property |
Worth |
|
chemical symbol |
OR |
|
Atomic number |
92 |
|
Atomic mass (U-238) |
Approximately 238.05078 u |
|
Physical state |
Solid (at room temperature) |
|
Important isotopes |
U-238, U-235 |
|
Melting point |
Approximately 1,132°C (2,070°F) |
|
Boiling point |
Approximately 3,818°C (6,904°F) |
|
Radioactive properties |
Emits alpha, beta and gamma radiation |
|
Toxicity |
Toxic to humans |
|
Applications |
Nuclear energy, nuclear weapons, radiometric dating |
|
Abundance |
Relatively rare, it is found in minerals such as uraninite and carnotite |
Uranium isotopes
Uranium can occur in different compositions in its nucleus, that is, in different isotopes. Although uranium can be found in nature, most of it is in a configuration that is not the most suitable for generating nuclear reactions.
For this reason, the atoms of this element are artificially altered to convert them into other, more unstable isotopes. These new isotopes will favor the generation of nuclear fission chain reactions.
Below are the most prominent isotopes:
-
Uranium-235 (U-235): It is the isotope of uranium that is used as fuel in nuclear reactors and for the manufacture of nuclear weapons. U-235 is fissile, meaning it can split into two lighter nuclei when it absorbs a neutron, releasing a large amount of energy in the form of heat and radiation. This process is fundamental in nuclear fission reactions and is the principle behind nuclear bombs and power generation in nuclear reactors.
-
Uranium-238 (U-238): It is the most abundant uranium isotope and makes up most of natural uranium. U-238 can also fission, but is not fissionable by thermal neutrons, making it less useful for most nuclear fission applications. However, U-238 is converted to plutonium-239 (Pu-239) when it absorbs a neutron, and Pu-239 is another fissile material used in the manufacture of nuclear weapons and as fuel in fission reactors.
-
Uranium-233 (U-233): Although less common, U-233 is another fissile isotope of uranium that can be used as fuel in nuclear reactors. It is formed from thorium-232 (Th-232) through the absorption of a neutron. Although it is an efficient fissionable, it is more complicated to produce and handle than U-235 or Pu-239.
In addition to the isotopes mentioned, there are other less common isotopes. These include uranium-234 (U-234), uranium-236 (U-236), uranium-239 (U-239), among others. These isotopes are less abundant and have more limited applications in nuclear technology due to their low prevalence.