
Pressure is the physical quantity that measures the force exerted on a unit of surface applied in a direction perpendicular to it. It can be calculated by dividing the applied force by the area over which that force is applied.
The mathematical formula for calculating pressure is:
Pressure = Force / Area
This physical quantity can have different effects depending on the context in which it is applied. For example, in fluid dynamics and mechanics in particular, pressure is related to properties such as the density and height of the fluid. Many physical phenomena such as flotation and the operation of fluids in pipes and hydraulic systems depend on pressure.
In the field of nuclear energy, the pressure in a nuclear reactor is important to ensure efficient heat transfer, control reactivity, maintain the integrity of the containment system, ensure safety and contribute to energy efficiency.
Types of pressure
There are the following types:
-
Hydrostatic pressure : the pressure exerted by non-compressible liquids on objects that are in contact with them.
-
Absolute pressure: is the sum of the force per unit of surface of a given system and that of the air that surrounds it.
-
Gauge pressure (also called relative pressure) is the difference between absolute (real) pressure and atmospheric pressure.
-
Atmospheric pressure : is the force per unit of surface that the air exerts on the Earth's surface. At sea level, 1 atm is equivalent to 101325 Pa.
-
Arterial or blood: when we refer to the force exerted by the blood on the internal surface of the arteries.
-
Osmotic: is the force exerted per unit surface of a solution against a closed semipermeable membrane.
Pressure units
The unit of pressure in the International System of Units is the pascal (Pa), named after Blaise Pascal. One pascal is the pressure exerted by a total force of one newton acting uniformly on one square metre (Pa = N/m2).
The Pascal (Pa) is a small unit, and sometimes it is convenient to use other units of measurement:
-
Millimeter of mercury (mmHg): is a unit that is still used to measure pressure in medicine, meteorology, aviation and is equivalent to 133.322 387 415 Pa.
-
bar. The use of this unit is accepted within the SI although it is not recommended. It is often used because it has a value very close to 1 atm. 1 bar = 100,000 Pa
-
Hectopascals (HPa): This unit is used in meteorology and is equivalent to 100 Pa.
-
Atmosphere (atm): The atmosphere is a unit that indicates the pressure that the Earth's atmosphere produces on average on the Earth's surface.
-
Kiloponds per square centimetre (kp/cm²): This unit is used in engineering. The kilopond is equal to the weight of a mass of one kilogram (9.8 N).
-
Pound-force per square inch (lbf/in²): is a unit belonging to the Anglo-Saxon system. The acronym psi (pounds-force per square inch) is also used to refer to it.
Devices for measuring pressure
 In physics, pressure is measured using a variety of instruments and techniques depending on the system or phenomenon being studied. Some examples are shown below:
In physics, pressure is measured using a variety of instruments and techniques depending on the system or phenomenon being studied. Some examples are shown below:
-
Manometers: These are used to measure pressure in gases and liquids. These instruments measure pressure based on the deformation of a pressure-sensitive element or by measuring the height of a liquid column in equilibrium with the pressure.
-
Barometers: Barometers are instruments specifically designed to measure atmospheric pressure.
-
Pressure Sensors: In more advanced applications, electronic pressure sensors are used that convert pressure into an electrical signal.
-
U-tubes: One end of the tube is connected to the system where pressure is to be measured, and the other end is either kept open or connected to a known reference point. The difference in height of the fluids on the two sides of the U-tube provides a measure of the pressure difference.
Pressure in liquids, solids and gases
The pressure in liquids, solids and gases behaves differently due to the intrinsic properties of each state of matter as you can see below:
Pressure in liquids
In liquids, pressure is transmitted isotropically, that is, in all directions equally according to Pascal's principle.
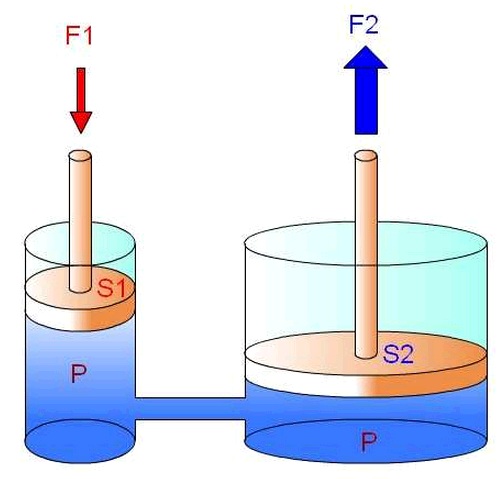 The pressure in a liquid depends on the depth at which it is located and the density of the liquid. At greater depths, the pressure increases due to the weight of the liquid above. This is due to hydrostatic pressure, which is the result of the force exerted by the weight of the liquid on a given area.
The pressure in a liquid depends on the depth at which it is located and the density of the liquid. At greater depths, the pressure increases due to the weight of the liquid above. This is due to hydrostatic pressure, which is the result of the force exerted by the weight of the liquid on a given area.
Hydrostatic pressure is calculated using the formula P = ρgh, where P is the pressure, ρ is the density of the liquid, g is the acceleration due to gravity, and h is the height or depth of the liquid.
Pressure on solids
In solids, pressure manifests itself as a force applied to a given surface. Pressure in a solid is the result of the distribution of the force over the contact area.
As the applied force increases, the pressure also increases. Pressure on solids can be calculated by dividing the applied force by the area over which that force is applied.
Pressure in gases
In gases, pressure is due to collisions between gas molecules and the walls of the container. The greater the number of molecules or the greater the kinetic energy of the molecules, the more collisions there will be and, therefore, greater pressure.
The pressure of a gas can be calculated using the ideal gas law, which states that pressure is directly proportional to the absolute temperature and the number of molecules present, and inversely proportional to the volume.
This law is expressed mathematically as P = nRT/V, where P is the pressure, n is the number of moles of gas, R is the ideal gas constant, T is the absolute temperature, and V is the volume of the gas.
Importance of pressure in a nuclear power plant
In a nuclear reactor, pressure control allows water to be kept in a liquid state at temperatures above 100°C, its normal boiling point at atmospheric pressure. For example, in a pressurized water reactor (PWR), water can be at a pressure of approximately 155 bar (15.5 MPa), allowing it to reach temperatures close to 300°C without boiling.
Pressure also affects the efficiency of converting thermal energy into electrical energy. In steam generators, high-pressure water produces steam with higher thermal energy. For example, in a typical nuclear power plant, steam can be generated at a pressure of around 70 bar (7 MPa), which improves turbine performance and increases electricity output.
Nuclear safety
 In addition, pressure is crucial in many aspects of the operation of a nuclear power plant for reactor safety. Below are two prominent examples:
In addition, pressure is crucial in many aspects of the operation of a nuclear power plant for reactor safety. Below are two prominent examples:
First, in a nuclear power plant, pressure is used to prevent radioactive particles from escaping to the outside. The reactor is contained within a robust structure that withstands high pressures, preventing the release of radioactive materials in the event of pressure increases. High-pressure cooling systems keep water in a liquid state at high temperatures, preventing it from turning into steam and potentially escaping.
Ventilation systems are equipped with high-efficiency filters that operate under differential pressure, capturing radioactive particles before they leave the plant. In certain areas, a lower pressure (depression) is maintained than outside, ensuring that air flows in in the event of leaks. In addition, joints and seals in the pipes are designed to withstand high pressures and can prevent leakage of radioactive materials.
The pressure on people
In the context of health, pressure commonly refers to blood pressure, which is the force exerted by blood against the walls of the arteries as the heart beats and relaxes. Blood pressure is expressed by two values: systolic pressure and diastolic pressure.
- Systolic pressure: This is the first value recorded when measuring blood pressure and represents the pressure in the arteries when the heart contracts and pumps blood to the body.
- Diastolic pressure: This is the second value recorded and represents the pressure in the arteries when the heart relaxes between beats.
Blood pressure is measured in millimeters of mercury (mmHg) and is expressed as a fraction, for example, 120/80 mmHg. The first number (systolic pressure) is higher because it is measured during contraction of the heart, while the second number (diastolic pressure) is lower because it is measured during relaxation of the heart.