
A chemical element is a pure substance with certain physical and chemical properties. The atomic nucleus of each element consists of protons and neutrons. The number of protons in the nucleus of an atom - atomic number - is unique for each chemical element.
Sometimes, some authors refer to a chemical species to refer to a certain atom. However, this term is also used to refer to other substances such as molecules, ions, etc.
Each chemical element has its Latin name and the chemical symbol consisting of one or two letters. The names and symbols of the elements are regulated by the IUPAC and are reflected in Mendeleev's periodic table of elements.
The form of existence of chemical elements in free form are simple substances. It is necessary to distinguish between chemical elements (abstract objects described through their characteristics) and the corresponding material objects: simple chemical compounds (with certain physical and chemical properties).
A particular group of elements are the noble gases. Noble gases are a group of chemical elements with very similar properties: they are odorless, colorless monatomic gases and have very low chemical reactivity.
Chemical elements can combine with each other to form another defined chemical composition through chemical reactions. For example, the element oxygen and hydrogen can combine to form water.
How many are the chemical elements?
118 chemical elements are known, of which 94 are found in nature (some are only found in small quantities). The remaining 24 are obtained artificially as a result of nuclear reactions.
Man-made chemical elements have a higher atomic number than uranium. Its synthesis was initially carried out by the repeated capture of neutrons by uranium nuclei under conditions of intense neutron flux in nuclear reactors.
Uranium isotopes are used to make nuclear fuel. They have a very unstable nature that makes them suitable for the nuclear energy industry.
What does the name of the elements depend on?
The right to propose a name for a new chemical element is granted to the discoverers. However, this name must adhere to certain rules. Publishing a new discovery is verified within a few years by independent laboratories. If the existence of the element is confirmed, the International Union of Pure and Applied Chemistry officially approves the name of the new element.
The 118 elements known as of December 2016 have permanent names approved by IUPAC. From the time of the opening request to the approval of the IUPAC name, the item appears under a temporary systematic name.
Unopened or unapproved elements are often named using the system used by Mendeleev, that is, by the name of the higher homolog in the periodic table. In this case, the prefixes "ek-" or (rarely) "dvi-" are added, which means Sanskrit numbers "one" and "two" depending on whether the homolog is 1 or 2 periods higher.
What are the chemical elements?
Chemical elements are classified on the periodic table of elements.
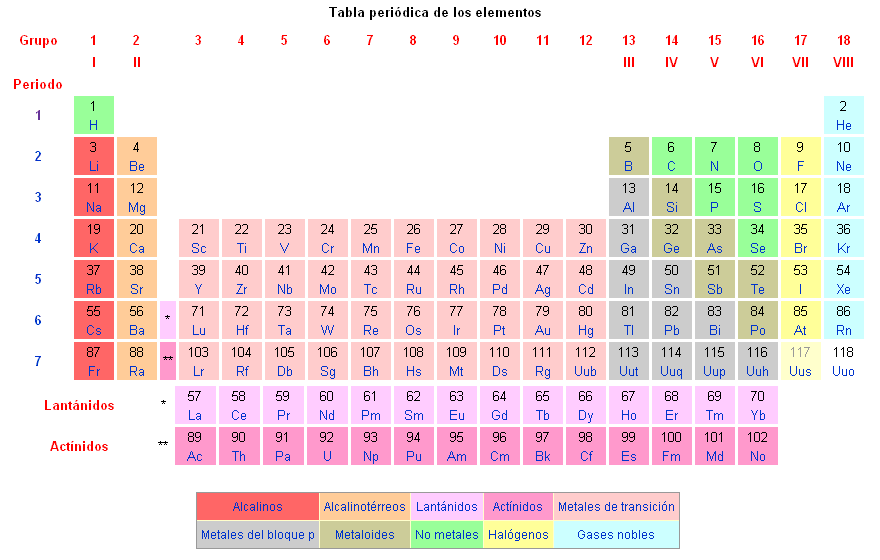
Known chemical elements are as follows:
| Chemical element | Symbol | Atomic number (Z) | Atomic weight (u) |
| Hydrogen | H | 1 | 1.0079 |
| Helio | He | 2 | 4.0026 |
| Lithium | Li | 3 | 6941 |
| Beryllium | Be | 4 | 9.0122 |
| Boro | B | 5 | 10811 |
| Carbon | C | 6 | 12.0107 |
| Nitrogen | N | 7 | 14.0067 |
| Oxygen | O | 8 | 15.9994 |
| Fluorine | F | 9 | 18.9984 |
| Neon | Ne | 10 | 20.1797 |
| Sodium | Na | 11 | 22.9897 |
| Magnesium | Mg | 12 | 24305 |
| Aluminum | Al | 13 | 26.9815 |
| Silicon | Si | 14 | 28.0855 |
| Match | P | 15 | 30.9738 |
| Sulfur | S | 16 | 32065 |
| Chlorine | Cl | 17 | 35453 |
| Argon | Ar | 18 | 39948 |
| Potassium | K | 19 | 39.0983 |
| Football | Ca | 20 | 40078 |
| Scandium | Sc | 21 | 44,9559 |
| Titanium | Ti | 22 | 47867 |
| Vanadium | V | 23 | 50.9415 |
| Chrome | Cr | 24 | 51,9961 |
| Manganese | Mn | 25 | 54938 |
| Iron | Fe | 26 | 55845 |
| Cobalt | Co | 27 | 58,9332 |
| Nickel | Ni | 28 | 58.6934 |
| Copper | Cu | 29 | 63546 |
| Zinc | Zn | 30 | 65.39 |
| Gallium | Ga | 31 | 69723 |
| Germany | Ge | 32 | 72.64 |
| Arsenic | As | 33 | 74.9216 |
| Selenium | Se | 34 | 78.96 |
| Bromo | Br | 35 | 79904 |
| Krypton | Kr | 36 | 83.8 |
| Rubidio | Rb | 37 | 85.4678 |
| Strontium | Sr | 38 | 87.62 |
| Yttrium | Y | 39 | 88,9059 |
| Zirconium | Zr | 40 | 91224 |
| Niobium | Nb | 41 | 92,9064 |
| Molybdenum | Mo | 42 | 95.94 |
| Technetium | Tc | 43 | 98 |
| Rutenio | Ru | 44 | 101.07 |
| Rodio | Rh | 45 | 102,9055 |
| Palladium | Pd | 46 | 106.42 |
| The payment | Ag | 47 | 107,8682 |
| Cadmium | Cd | 48 | 112411 |
| Indian | In | 49 | 114818 |
| Tin | Sn | 50 | 118.71 |
| Antimony | Sb | 51 | 121.76 |
| Teluro | Te | 52 | 127.6 |
| Iodine | I | 53 | 126.9045 |
| Xenon | Xe | 54 | 131293 |
| Cesium | Cs | 55 | 132.9055 |
| Barium | Ba | 56 | 137327 |
| Lantano | La | 57 | 138.9055 |
| Cerium | Ce | 58 | 140116 |
| Praseodimio | Pr | 59 | 140.9077 |
| Neodymium | Nd | 60 | 144.24 |
| Prometheus | Pm | 61 | 145 |
| Samaria | Sm | 62 | 150.36 |
| Europe | Eu | 63 | 151964 |
| Gadolinium | Gd | 64 | 157.25 |
| Terbio | Tb | 65 | 158,9253 |
| Disprosio | Dy | 66 | 162.5 |
| Holmio | Ho | 67 | 164,9303 |
| Erbio | Er | 68 | 167259 |
| Thulium | Tm | 69 | 168.9342 |
| Iterbio | Yb | 70 | 173.04 |
| Lutecio | Lu | 71 | 174967 |
| Shaping | Hf | 72 | 178.49 |
| Tantalum | Ta | 73 | 180,9479 |
| Tungsten | W | 74 | 183.84 |
| Renio | Re | 75 | 186207 |
| Osmio | Os | 76 | 190.23 |
| Iridium | Ir | 77 | 192217 |
| Platinum | Pt | 78 | 195078 |
| Oro | Au | 79 | 196.9665 |
| Mercury | Hg | 80 | 200.59 |
| Thallium | Tl | 81 | 204.3833 |
| Lead | Pb | 82 | 207.2 |
| Bismuth | Bi | 83 | 208,9804 |
| Polonium | Po | 84 | 209 |
| Astato | At | 85 | 210 |
| Radon | Rn | 86 | 222 |
| France | Fr | 87 | 223 |
| Radio | Ra | 88 | 226 |
| Actinio | Ac | 89 | 227 |
| Thorium | Th | 90 | 232.0381 |
| Protactinio | Pa | 91 | 231.0359 |
| Uranium | U | 92 | 238.0289 |
| Neptune | Np | 93 | 237 |
| Plutonium | Pu | 94 | 244 |
| Americio | Am | 95 | 243 |
| Curio | Cm | 96 | 247 |
| Berkelio | Bk | 97 | 247 |
| Californio | Cf | 98 | 251 |
| Einstenio | Es | 99 | 252 |
| Fermio | Fm | 100 | 257 |
| Mendelevio | Md | 101 | 258 |
| Nobel | No | 102 | 259 |
| Laurencio | Lr | 103 | 262 |
| Rutherfordio | Rf | 104 | 261 |
| Dubnio | Db | 105 | 262 |
| Seaborgio | Sg | 106 | 266 |
| Bohrio | Bh | 107 | 264 |
| Hassio | Hs | 108 | 277 |
| Meitnerio | Mt | 109 | 268 |
| Darmstadtio | Ds | 110 | 281 |
| Roentgenio | Rg | 111 | 272 |
| Copernicus | Cn | 112 | 285 |
| Nihonio | Nh | 113 | 286 |
| Flerovio | Fl | 114 | 289 |
| Moscow | Mc | 115 | 288 |
| Livermorio | Lv | 116 | 292 |
| Teneso | Ts | 117 | 294 |
| Oganesón | Og | 118 | 294 |
How is a chemical element presented?
Chemical element symbols are used as abbreviations for the name of the elements. As a symbol, they usually take the initial letter of the item's name and, if necessary, add the next or one of the following. These are usually the initial letters of the Latin names of the elements.
 Such a system of chemical symbols was proposed in 1814 by the Swedish chemist J. Berzelius. The elements used before the official approval of their names and permanent symbols consist of three letters, which means that the Latin names of the three digits in the decimal notation of their atomic number. The notation system for higher homologues described above (Eka-Rn, Eka-Pb, etc.) is also used.
Such a system of chemical symbols was proposed in 1814 by the Swedish chemist J. Berzelius. The elements used before the official approval of their names and permanent symbols consist of three letters, which means that the Latin names of the three digits in the decimal notation of their atomic number. The notation system for higher homologues described above (Eka-Rn, Eka-Pb, etc.) is also used.
The smaller numbers next to the element symbol indicate:
-
The atomic mass in the upper left.
-
Atomic number in the lower left.
