
The field of nuclear energy is vast and complex, encompassing a wide range of technical concepts and applications. This article provides a short glossary of basic concepts with clear and concise definitions of key terms related to nuclear energy.
From the fundamental processes of fission and fusion to concepts associated with safety, waste management, and practical applications, each term is explained to provide a solid foundation of knowledge.
What is the nuclear energy?
Nuclear energy is a way of obtaining electricity by harnessing the energy stored in the nuclei of atoms. In nuclear power plants, a process called nuclear fission takes place, in which the nuclei of atoms such as uranium are divided into smaller parts. This process releases a large amount of heat, which is used to heat water and produce steam to drive a turbine connected to a generator. In this way, the energy of the atoms is converted into electricity.
In addition to generating electricity, nuclear energy has other important uses. For example, it is used in medicine for diagnosis and treatment, in scientific research to study materials, and in industry to irradiate food and control the quality of materials. On the other hand, it is also used in the propulsion of submarines and aircraft carriers and even for the creation of bombs with great destructive power.
Nuclear reactions: fission and fusion
In order to take advantage of the nuclear energy present in the nucleus of atoms, there are two ways to do it: splitting the nucleus of an atom or fusing the nuclei of two atoms. In the first case, we call it nuclear fission and in the second, nuclear fusion.
When one of these two physical reactions occurs, the atoms experience a slight loss of mass. This lost mass is converted into a large amount of heat energy, as Albert Einstein discovered with his famous equation E=mc².
Technique 1: Nuclear fission
 Nuclear fission is a way of obtaining the energy contained in an atom by dividing the atomic nucleus into different smaller particles. This type of reaction generates a large amount of heat energy that can later be used in different ways.
Nuclear fission is a way of obtaining the energy contained in an atom by dividing the atomic nucleus into different smaller particles. This type of reaction generates a large amount of heat energy that can later be used in different ways.
One of the important characteristics of nuclear fission is that it is generated by bombarding an unstable atom with a neutron. Once the nucleus has fissioned, in addition to particles, one or two more neutrons are released that can collide with other atoms, generating a chain reaction.
The resulting particles are considered spent fuel that must be replaced when the ratio becomes too high and prevents free neutrons from finding atoms to fission.
Nuclear fission is currently the type of nuclear reaction used in all types of nuclear power reactors. Most of these reactors are light water reactors.
Technique 2: Nuclear fusion
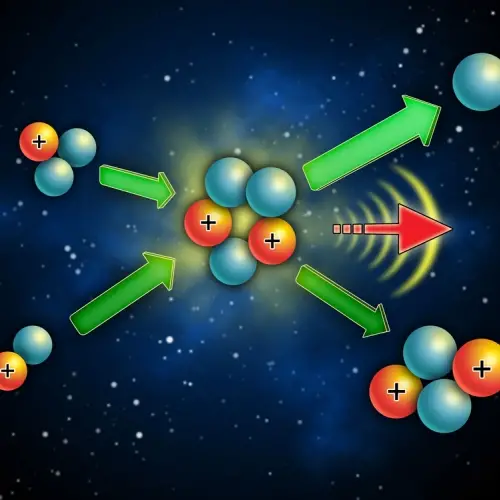 Nuclear fusion is the reverse process, that is, the fusion of the nuclei of two atoms. To achieve fusion, the nuclei of the atoms must be subjected to very high pressure and temperature conditions. This type of reaction also produces a large amount of energy.
Nuclear fusion is the reverse process, that is, the fusion of the nuclei of two atoms. To achieve fusion, the nuclei of the atoms must be subjected to very high pressure and temperature conditions. This type of reaction also produces a large amount of energy.
However, technically viable nuclear power reactors for electricity production have not yet been developed.
This technique has multiple advantages compared to fission:
- It is more sustainable for the environment
- Better performance
- It would be a renewable source of energy.
An example of nuclear fusion energy is the energy produced by the Sun. Nuclear fusion reactions occur in the core of the star in our Solar System.
Other concepts and definitions
- Atom: The basic unit of matter, composed of a nucleus (protons and neutrons) and a cloud of electrons.
- Neutron: Subatomic particle without electrical charge, present in the nucleus of atoms, which plays a crucial role in nuclear reactions.
- Isotope: Variants of a chemical element that have the same number of protons but a different number of neutrons.
- Radioactivity: Spontaneous emission of radiation by unstable nuclei of atoms. It may include alpha, beta particles, and gamma rays, and is a key phenomenon in nuclear fission and fusion.
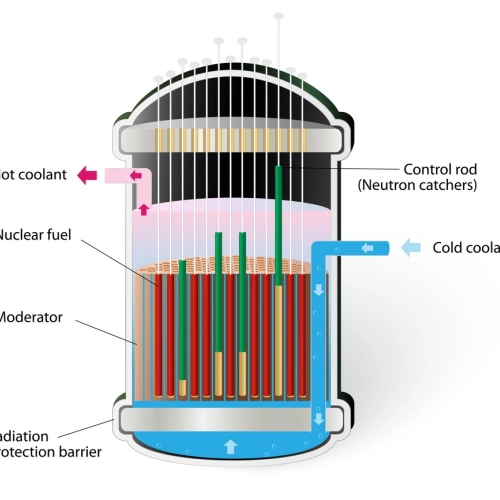 Nuclear reactors: Devices that initiate and control the fission reaction to generate heat, which is converted into electricity.
Nuclear reactors: Devices that initiate and control the fission reaction to generate heat, which is converted into electricity.- Pressurized Water Reactors (PWR): A type of reactor that uses high-pressure water to prevent boiling, and transferring heat to a separate steam system.
- Boiling water reactors (BWR): A type of reactor in which water boils in the core to directly generate steam, which is used to drive a turbine.
- Nuclear fuel: Material used in nuclear reactors to generate energy through fission. Common fuels include uranium-235 and plutonium-239. These materials release energy by splitting into lighter nuclei.
- Fissile material: Material that can sustain a fission chain reaction, such as uranium-235 or plutonium-239.
- Reaction chain: Series of nuclear reactions in which the released neutrons induce the fission of other nuclei.
- Fuel rods: Components in a nuclear reactor that contain fissile material and are used to sustain the fission reaction.
- Moderator: Material that reduces the speed of neutrons to facilitate continuous fission in a nuclear reactor.
- Coolant: A substance that circulates through the reactor to absorb and transfer heat generated by fission.
- Radioactive waste: Materials that contain radioactive isotopes and require safe handling and storage due to their radioactivity.
- Nuclear power plant: A facility that uses nuclear reactors to generate electricity through the process of nuclear fission. It consists of one or more reactors, steam-generating systems, and energy conversion equipment.
- Containment building: Structure designed to confine fission products and prevent the release of radioactive materials into the environment.
- Decommissioning: The process of safely deactivating and disposing of a nuclear power plant at the end of its useful life.
- Nuclear accident: An incident at a nuclear power plant that results in an unexpected release of radioactive materials into the environment, potentially with serious consequences for human health and the environment. Examples include Chernobyl and Fukushima.
- Nuclear medicine: A branch of medicine that uses radioactive isotopes to diagnose and treat disease. Procedures include positron emission tomography (PET) imaging and radionuclide therapy.
- Atomic bomb: A nuclear weapon that uses the fission of heavy atoms, such as uranium or plutonium, to release a huge amount of explosive energy. The first atomic bomb was dropped on Hiroshima in 1945.
- Hydrogen bomb: A nuclear weapon that uses the fusion of light atoms, such as deuterium and tritium, to release much greater energy than an atomic bomb. It is also known as a thermonuclear bomb.